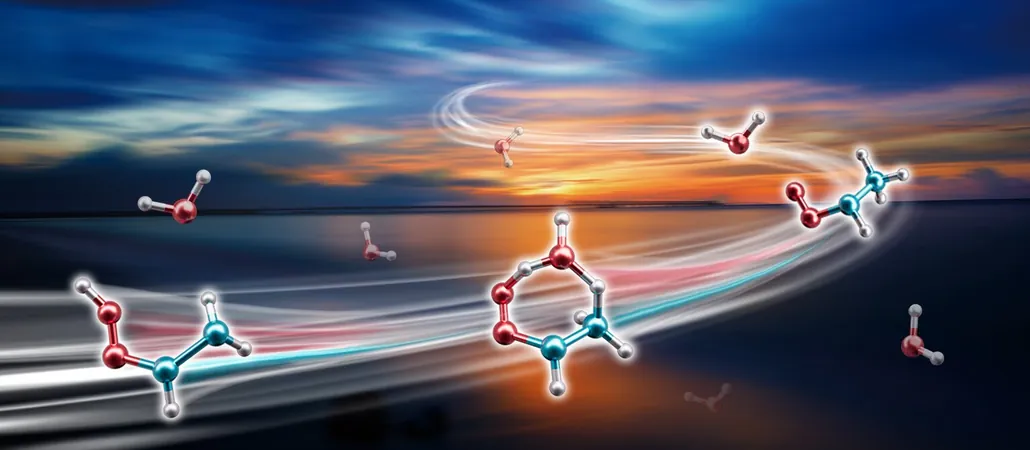
Scientists Discover Shocking Speed of Atmospheric Reaction with Criegee Intermediates!
2025-04-28
Author: Michael
A Major Breakthrough in Atmospheric Chemistry!
In a groundbreaking study, researchers have unveiled that Criegee intermediates (CIs)—the highly reactive chemicals formed when ozone interacts with alkenes—react with water vapor in the atmosphere much more rapidly than previously thought.
What Are Criegee Intermediates?
These intermediates, particularly syn-CH3CHOO, are pivotal in generating hydroxyl radicals, known as the atmosphere's "cleaning agents," and in forming aerosols that significantly influence climate and air quality. Surprisingly, syn-CH3CHOO accounts for a staggering 25% to 79% of all CIs, depending on the season.
The Unexpected Speed Surge!
Traditionally, scientists believed that syn-CH3CHOO primarily vanished through self-decomposition. However, a team from the Dalian Institute of Chemical Physics has discovered a startling new reaction pathway! According to their findings published in Nature Chemistry, syn-CH3CHOO's reaction with atmospheric water vapor is approximately 100 times faster than theoretical models indicated.
Innovative Research Techniques!
Using state-of-the-art laser techniques, the researchers could accurately measure the reaction rate between syn-CH3CHOO and water vapor, leading to this astonishing revelation. To delve deeper into the mechanics behind this rapid reaction, they developed a highly accurate full-dimensional potential energy surface via advanced neural network approaches and conducted thorough dynamical calculations.
Discovering the "Roaming Mechanism"!
The research unveiled a fascinating "roaming mechanism" fueled by strong dipole-dipole interactions between the molecules. Instead of adhering to a straightforward energy path, these molecules maneuver closely together, significantly upping the chances of a reaction. Under typical atmospheric conditions, this water-mediated reaction pathway is just as crucial as self-decomposition, which has long been assumed to dominate.
Implications for Climate Science!
This revolutionary discovery challenges the prevailing notion that unimolecular decomposition is the primary way syn-CH3CHOO is removed from the atmosphere. By reshaping our understanding of these fundamental atmospheric processes, scientists could refine models predicting climate change and air quality. This study also emphasizes the critical need to blend high-precision experimental data with sophisticated simulations to accurately forecast intricate chemical reactions.








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)