Revolutionary Discovery in Amyloid Beta Fibril Growth Paves the Way for Alzheimer’s Treatment!
2024-10-24
Author: Yu
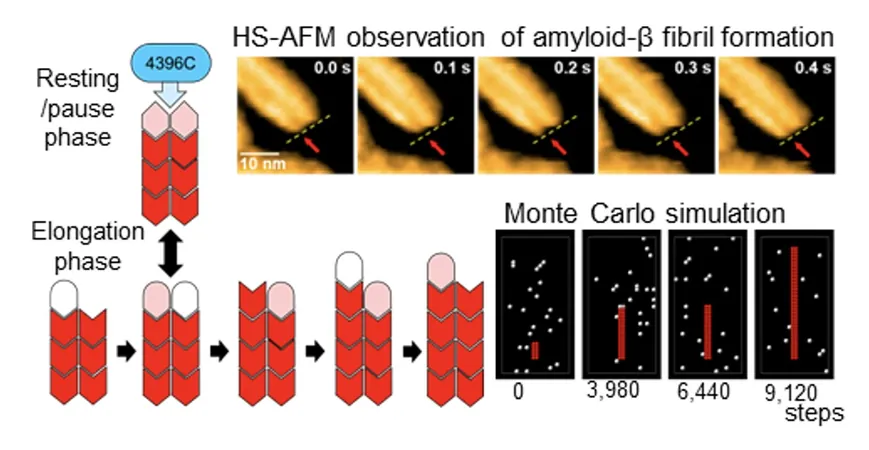
In an exciting breakthrough for Alzheimer’s research, a collaborative team of scientists has unveiled a new mechanism behind the growth of amyloid beta (Aβ) fibrils, which play a significant role in the progression of this debilitating disease. Utilizing cutting-edge high-speed atomic force microscopy (HS-AFM), researchers observed Aβ fibril formation at the molecular level in real time, as documented in the Journal of the American Chemical Society.
This significant advance shines a light on the intricate process of fibril growth and offers potential strategies to halt its progression, raising hopes in the fight against Alzheimer’s disease—an insidious neurodegenerative disorder leading to cognitive decline and memory loss.
Aβ proteins accumulate in the brain and aggregate to form harmful fibrils that disrupt normal brain function. The exact mechanisms of Aβ fibril growth have long been a mystery, but this study has cracked the code.
The multidisciplinary research group, which includes esteemed teams from the Exploratory Research Center on Life and Living Systems, the Institute for Molecular Science of the National Institutes of Natural Sciences, and several prominent Japanese universities like Nagoya City University, Nagoya University, and the University of Tsukuba, discovered that each Aβ fibril consists of two slender strands known as protofilaments. These strands develop in an alternating manner, with individual Aβ molecules attaching to the ends one at a time.
A pivotal finding in this study is the identification of a "paused state," where the ends of the two protofilaments align, causing a temporary halt in fibril growth. This paused state is not only essential to fibril formation but also provides a crucial insight into how Alzheimer’s disease may progress.
Moreover, the research highlighted the remarkable role of a specific antibody, 4396C, which binds selectively to the ends of Aβ fibrils during this paused state. Once attached, the antibody effectively locks the fibril in a non-growing state, offering a promising avenue for therapeutic intervention. This compelling discovery could lead to innovative treatments that slow down or even halt the progression of Alzheimer’s disease.
The exceptional high-resolution observations enabled by HS-AFM have propelled our understanding of the alternating growth mechanism and the strategic paused state, opening up new frontiers for treatment options. The implications could extend beyond Alzheimer’s, offering potential strategies for tackling various amyloid-related diseases, where protein aggregation is a common factor.
Looking to the future, researchers are eager to delve deeper into the functionality of the 4396C antibody and its potential for therapeutic application. This pioneering study reflects the relentless drive to develop effective treatments that may ultimately change the landscape of Alzheimer’s disease and similar conditions that involve protein-related issues.
Stay tuned as the team continues its groundbreaking work, paving the way for hope where there was once despair in the fight against Alzheimer’s.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)