Revolutionary AI Tools Unlock Secrets of Enzyme Mechanisms for Groundbreaking Lasso Peptide Drugs!
2024-09-20
In a groundbreaking study published in *Nature Chemical Biology*, researchers have harnessed the power of artificial intelligence to unravel the mechanisms behind lasso peptides—unique natural products synthesized by bacteria. With their rare lasso structure, these peptides exhibit remarkable stability, making them incredibly resilient to extreme environments. Their potential as therapeutic agents is immense, particularly in the realms of antibacterial, antiviral, and anti-cancer applications.
"Lasso peptides are fascinating because they are linear molecules that essentially get tied into a slip knot-like form," explained Susanna Barrett, a graduate student at the Mitchell lab. This unconventional structure not only enhances their stability but also allows for greater engineering versatility in drug development.
These remarkable peptides are produced through a process called ribosomal synthesis, which is followed by a series of post-translational modifications. During this process, a peptidase and a cyclase enzyme work together to convert a linear precursor peptide into the characteristic knotted form. Since the discovery of lasso peptides over three decades ago, scientists have grappled with understanding the precise mechanics of how cyclases fold these intricate knots.
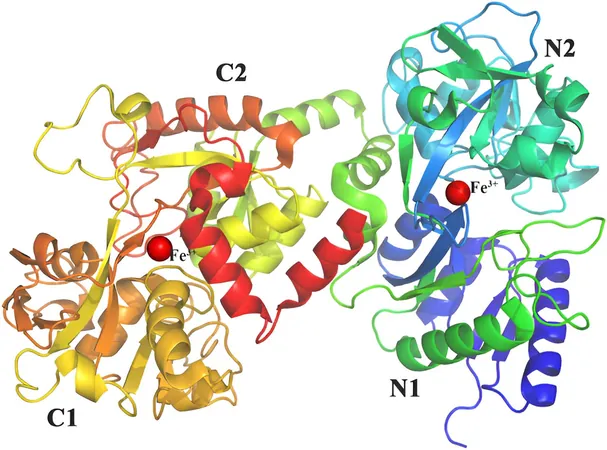
"The challenge lies in the fact that these enzymes are notoriously difficult to purify and work with, often remaining insoluble or inactive," Barrett noted. However, an exception emerged in the form of fusilassin cyclase, or FusC, which was successfully characterized by the Mitchell lab in 2019. Despite this advancement, the structure of FusC had remained a mystery, leaving significant gaps in understanding how it interacts with lasso peptides to facilitate their folding.
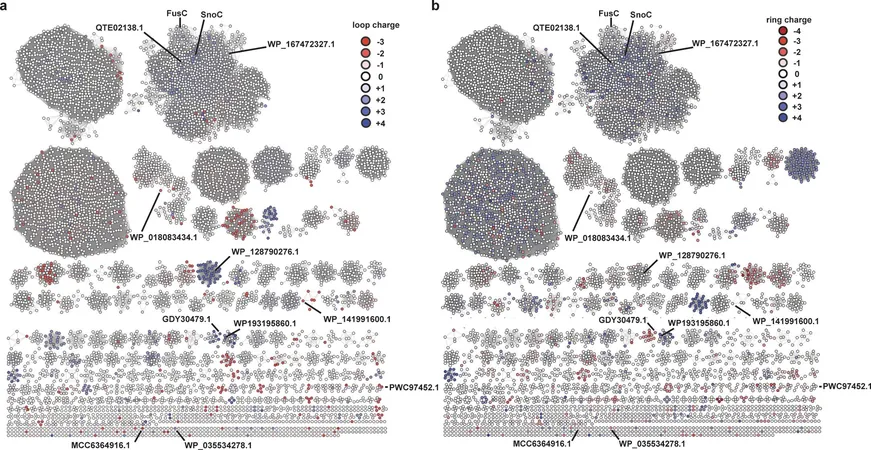
Leveraging the revolutionary AI program AlphaFold, researchers made significant progress in predicting the structure of the FusC protein. By combining this insight with other AI tools like RODEO, they identified crucial active site residues in the cyclase that play a pivotal role in interacting with the lasso peptide substrate.
According to Barrett, "FusC consists of approximately 600 amino acids, and its active site features 120. Utilizing these advanced programs allowed us to conduct structural studies and narrow down the important amino acids within the enzyme’s active site."
The team also employed molecular dynamics simulations through the powerful Folding@home platform, enabling them to visualize atomic-level interactions during the lasso folding process. "Prior to this study, there were no simulations focused on the relationships between lasso peptides and cyclases, so we believe our findings will be highly applicable in future peptide engineering endeavors," remarked Song Yin from the Shukla lab.
The researchers discovered that specific regions within the active site, particularly in FusC's helix 11 area, are crucial for the folding process. By conducting cell-free biosynthesis experiments, they introduced enzyme variants with different amino acids in this region and successfully identified a mutation that allowed the cyclase to fold lasso peptides not previously achievable by the original enzyme. This confirms the structural model they developed via computational methods.
"This study provides a first glimpse into the physical interactions responsible for generating these fascinating structures," stated Diwakar Shukla, an associate professor in chemical and biomolecular engineering. The findings suggest that these molecular interactions are consistent across different cyclases in various bacterial phyla, indicating a potentially universal model for lasso peptide folding.
In collaboration with Lassogen, a company based in San Diego, the researchers demonstrated how their insights could inform cyclase engineering to produce previously unattainable lasso peptides. As a proof of concept, they engineered another cyclase, McjC, to create a potent inhibitor targeting a cancer-promoting integrin.
"The ability to produce diverse lasso peptides is crucial for optimizing drug development," remarked Mark Burk, CEO of Lassogen. "Nature’s enzymes do not always facilitate the production of the lasso peptides we desire, and engineering these cyclases greatly enhances the therapeutic potential of these extraordinary molecules."
The implications of this research are immense, paving the way for new drug therapies derived from lasso peptides and demonstrating the transformative role AI can play in biotechnology. Stay tuned as the world of lasso peptides continues to unfold, potentially revolutionizing medicine as we know it!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)