Breakthrough in Industrial Lactoferrin Production: How Synthetic Biology Could Change the Game!
2024-09-25
Author: Mei
Lactoferrin (LF), a remarkable glycoprotein found in human and cow milk, is renowned for its ability to bind iron, which is crucial for various biological functions. Discovered in the 1930s, LF's multifaceted properties have intrigued scientists for decades. Its unique functions support infant health and development, bolster immune response, exhibit antibacterial and antiviral activities, and mitigate inflammation, making it a powerhouse ingredient in the food, cosmetic, and pharmaceutical industries.
Despite its promising applications, industrial production of LF faces significant hurdles. Traditional methods of isolating LF from milk often fall short of meeting the soaring global demand. To address this shortfall, researchers have turned to synthetic biological systems—genetically engineered microorganisms capable of manufacturing large quantities of LF.
A pivotal study published on August 20, 2024, in BioDesign Research, spearheaded by Dr. Kun Liu from Anhui Polytechnic University in China, sheds light on the design and optimization of LF expression systems. Dr. Liu emphasizes, "By utilizing synthetic biological expression systems, we can efficiently produce LF on an industrial scale, and the resulting structure is remarkably similar to that of naturally occurring LF."
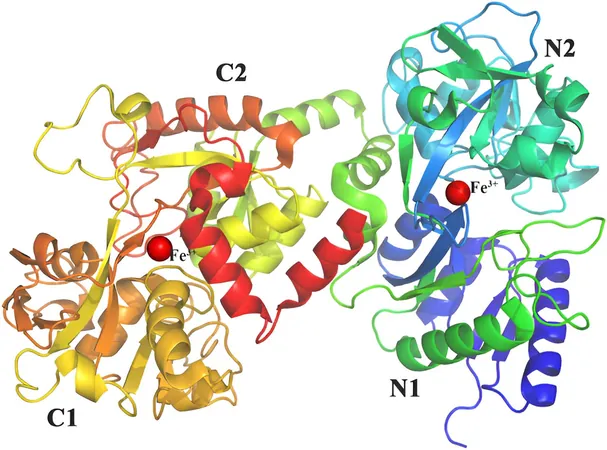
The successful synthesis of LF requires a thorough understanding of its molecular structure. Weighing in at around 80 kilodalton and composed of approximately 700 amino acids, LF features C- and N-terminal lobes, with the N-terminal lobe hosting the crucial iron-binding site. Scientists have found that enhancing the stability of this region, such as by adding disulfide bridges, can significantly improve LF resilience.
Moreover, LF undergoes a vital transformation known as N-linked glycosylation, where carbohydrates attach to its nitrogen atoms, enhancing its resistance to enzyme degradation. Achieving stability relies heavily on the choice of the expression host.
The study details four synthetic host systems: bacteria, yeast, filamentous molds, and mammalian cell lines, each with its advantages and challenges in LF production. Of these, E. coli remains the prominent choice; however, it struggles with protein degradation and lacks the requisite biochemical modification capabilities, which are critical for functional LF.
Yeast and mold systems present themselves as competitive alternatives, offering superior expression and processing capabilities, though they have toxicity issues that complicate production. The final option, cell lines, promises the most natural forms of LF but comes with high costs, contamination risks, and the potential for harboring human pathogens.
To tackle these challenges, researchers like Zhen Tong recommend redesigning transport mechanisms within expression hosts to enhance LF secretion while considering knockout strategies for harmful degradation enzymes.
The implications of these advancements in synthetic biology cannot be overstated. By honing in on efficient and reliable LF production methods, we stand on the brink of transforming healthcare and nutrition markets. Imagine a future where high-quality, sustainably produced lactoferrin is readily available, enhancing product formulations in food, healthcare, and beyond!
Stay tuned as science continues to unveil the potential of synthetic biology—and the next revolution in lactoferrin production could just be around the corner!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)