Groundbreaking Mouse Study Unveils Secrets of Nerve Cell Repair – A New Era for Treatments?
2024-09-27
Recent research emerging from The Ohio State University Wexner Medical Center and Imperial College London has made significant strides in understanding how nerve cells repair themselves, a discovery that could revolutionize treatments for nerve injuries. This pioneering study, published in the Proceedings of the National Academy of Sciences, focuses on the intricate 3D structure of DNA within nerve cells and its impact on healing post-injury.
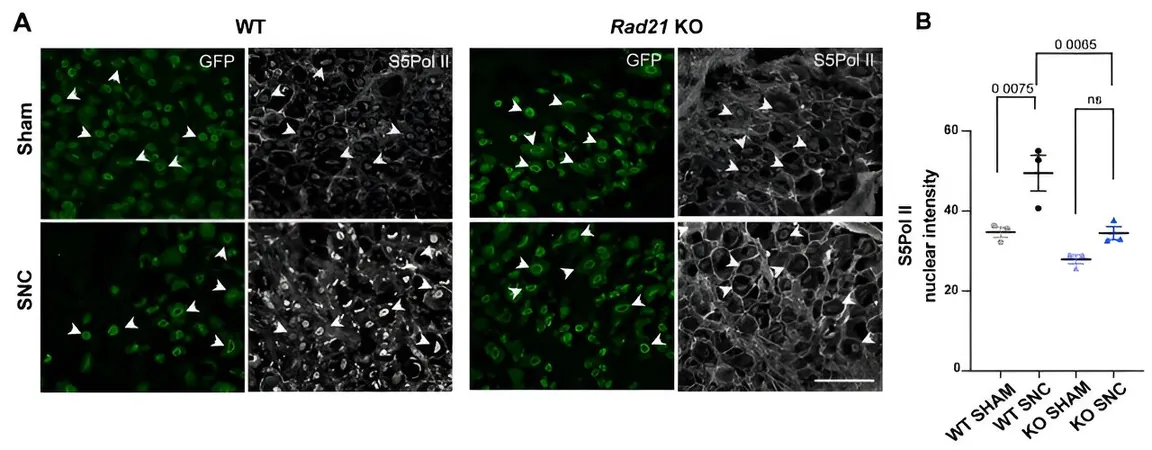
The research team, including lead author Ilaria Palmisano, Ph.D., uncovered that certain DNA configurations known as promoter-enhancer loops are crucial for activating genes essential for nerve repair. These DNA loops are stabilized by a protein complex called cohesin, which plays a pivotal role in the healing process.
"When cohesin is absent, the DNA loops fail to form appropriately, inhibiting the nerves' ability to heal," Palmisano explained. This critical insight into the mechanics of nerve regeneration could lead to innovative therapeutic strategies for treating nerve damage.
Collaboration was key in this study, with contributions from Professor Simone Di Giovanni and fellow scientists at the University of Miami. Their research builds on previous findings that demonstrated the significance of chromatin – the cell's 'instruction manual' – in nerve regeneration. The way chromatin unfolds and reorganizes directly influences how genes are expressed, which is vital for nerve repair.
Di Giovanni emphasized, "For neurons to regenerate effectively in the peripheral nervous system, it is essential for regenerative genes to be activated, leading to the production of proteins necessary for repairing injured nerves." This groundbreaking revelation will guide future research on enhancing cell repair mechanisms, especially in conditions where damage persists, such as in the central nervous system.
The study's findings suggest that after nerve injury, specific interactions occur between regenerative genes and their enhancers, indicating a complex communication network within the neuron. "Our study not only sheds light on neuronal biology but also opens new avenues for developing advanced repair strategies," Palmisano remarked.
Looking ahead, the researchers aim to delve deeper into how cohesin is activated following nerve injuries. Enhancing this activation could lead to groundbreaking applications in regenerative medicine, particularly in improving nerve recovery where traditional methods have fallen short.
As we stand on the brink of a new understanding of nerve cell repair, the implications of this research extend far beyond the lab, potentially ushering in novel treatments that could improve the quality of life for countless individuals suffering from nerve injuries. Could this be the key to unlocking superior healing solutions? Only time will tell.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)