Exciting Breakthrough: Scientists Find New Pathway to Create Superheavy Elements!
2024-10-27
Author: Arjun
What lies beyond the known elements of the periodic table?
As we journey into the depths of scientific discovery, researchers have unveiled a promising new method to synthesize superheavy elements—those elusive atoms with extraordinarily high atomic numbers that could unlock the mysteries of the universe!
Current Status of Superheavy Elements
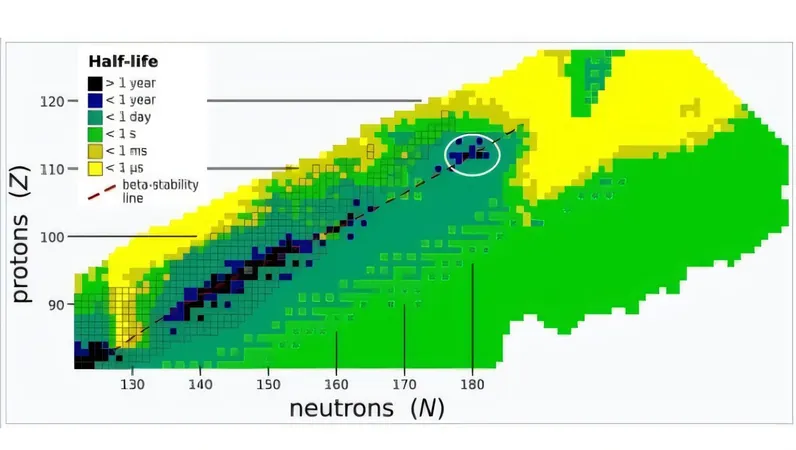
Currently, uranium holds the title for the heaviest abundant element, possessing 92 protons. However, scientists have successfully created superheavy elements, culminating in oganesson, which boasts an impressive 118 protons. Just below it are livermorium (116 protons) and tennessine (117 protons). Unfortunately, these superheavy elements exhibit notoriously brief half-lives, often decaying in less than a second, making their production and detection a complex endeavor that requires advanced particle accelerators.
Groundbreaking Development at Lawrence Berkeley National Laboratory
In a groundbreaking development, researchers from the United States and Europe have proposed an innovative method to push the boundaries of superheavy element production at the Lawrence Berkeley National Laboratory in California. Their findings, recently published in Physical Review Letters, mark a significant advancement in the field of nuclear physics.
The Island of Stability
Leading the study, J.M. Gates and colleagues introduced the concept of the "island of stability." This idea refers to a theoretical region in the nuclear landscape where superheavy elements may exhibit significantly longer half-lives than adjacent elements, notably expected around atomic number 112. Delving into this intriguing area could potentially revolutionize our understanding of nuclear stability and the formation of new elements.
Traditional Methods for Creating Superheavy Elements
Traditionally, creating these superheavy elements involved bombarding actinide targets—elements that range from atomic numbers 89 to 103—with beams of 48-calcium, a special isotope known for its "magic number" of protons and neutrons that makes it exceptionally stable. However, as researchers have sought to reach the island of stability, the existing methods have begun to plateau, necessitating a fresh approach.
New Approach: Heavier Isotopes
Gates and his team identified that heavier isotopes, specifically beams of 50-titanium, could be the key to producing new elements with atomic numbers 119 and 120. Their experimental work involved directing a high-intensity beam of titanium ions at a plutonium target. Over a 22-day period, the researchers successfully generated livermorium isotopes via two distinct nuclear decay chains.
Significance of the Achievement
This achievement is monumental—it demonstrates that collisions involving non-magic nuclei can lead to the creation of superheavy elements. Gates noted, "This is the first reported production of a superheavy element near the predicted island of stability with a beam other than 48-calcium." The implications are vast: this method could pave the way for further exploration and discovery of new superheavy elements, sparking excitement within the scientific community.
Implications for Nuclear Physics
The hunt for new isotopes is not just about filling gaps in the periodic table; it could have profound implications for our understanding of atomic and nuclear physics. Currently, approximately 110 isotopes of superheavy elements are known, but research suggests that up to 50 more could be lurking in the shadows, eagerly awaiting revelation through innovative techniques like those developed in this study.
Conclusion
As scientists continue to push the frontiers of chemistry and physics, this discovery marks yet another thrilling chapter in our quest to comprehend the universe's building blocks. What's next for these pioneering researchers? Only time will tell, but the possibilities are as boundless as the elements themselves!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)