Revolutionizing Lactoferrin Production: The Future of Synthetic Biological Systems
2024-09-25
Introduction
Lactoferrin (LF), a remarkable glycoprotein found in human and cow milk, has garnered significant attention since its discovery in the 1930s. Known for its iron-binding capabilities and multifaceted biological functions, LF is more than just a protein; it plays a crucial role in promoting infant health, enhancing immune responses, and exhibiting antibacterial and antiviral properties.
The Power of Lactoferrin
LF acts as a powerful agent in the human body, contributing to the innate immune response and serving as a frontline defender against bacterial and viral intrusions. By binding to iron, LF effectively limits bacterial growth while also enhancing the activity of immune cells such as macrophages and lymphocytes, making it an invaluable component in multiple industries, from food and cosmetics to pharmaceuticals.
The Industry Challenge
Despite its numerous benefits, the extraction and purification of LF from milk have proven to be problematic, leading to an inability to meet the surging market demand. Enter synthetic biological systems: a breakthrough in biotechnology that leverages microorganisms engineered through genetic techniques to mass-produce LF. This innovation marks a significant shift in how LF can be manufactured, making it more accessible and cost-effective.
Cutting-Edge Research
A recent study published on August 20, 2024, in *BioDesign Research* by a team at Anhui Polytechnic University, led by Dr. Kun Liu, reveals promising advancements in the design of LF expression systems. The research showcases a comprehensive analysis of the methodologies and challenges surrounding high-yield LF production.
Dr. Liu emphasizes that “the challenge in obtaining LF for market needs can be overcome by producing LF on an industrial scale through the use of synthetic biological expression systems.” These systems can produce LF that closely resembles its natural counterpart, ensuring functionality and effectiveness.
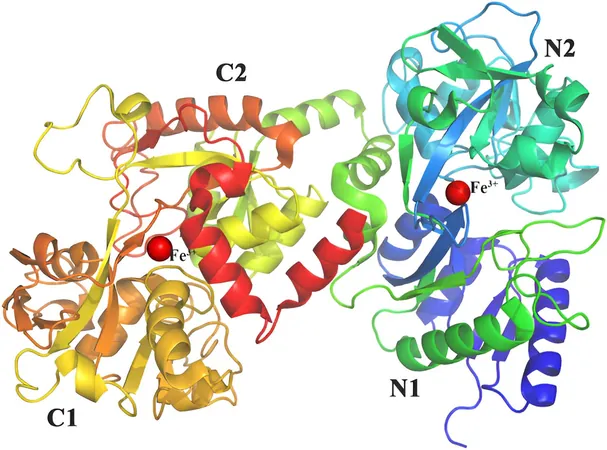
Understanding Lactoferrin's Structure
To engineer LF successfully, understanding its molecular structure is essential. With a weight of approximately 80 kilodaltons and composed of around 700 amino acids, LF contains distinct lobes that play crucial roles in its stability and function. The N-terminal lobe, housing the iron-binding site, is thermodynamically less stable, which researchers are working to enhance by introducing additional disulfide bridges.
Another critical aspect is the protein's glycosylation, which improves resistance to enzymatic degradation. The choice of host organism is paramount to achieving high stability and yield in LF production.
Various Synthetic Host Systems
The study delineates four primary systems for LF production: bacteria, yeast, filamentous molds, and mammalian cell lines.
1. **Bacterial Systems**: E. coli is frequently used due to its capacity to produce LF, reaching levels of 700 mg/L. However, issues related to proteolytic activity and inadequate biochemical modification machinery pose challenges.
2. **Yeast and Molds**: These host systems show promise due to their ability to perform essential modifications and produce stable LF. Yet, toxicity from the LF produced can inhibit expression levels, requiring innovative solutions to mitigate this risk.
3. **Cell Lines**: Although capable of producing LF that mirrors natural forms closely, challenges such as high cultivation costs and contamination risks hinder their widespread use.
Innovative Solutions Ahead
To address the challenges of synthetic biological systems, researchers advocate for redesigning transport mechanisms within host organisms to enhance LF secretion. Altering or knocking out enzymes that degrade LF can significantly improve yields.
Conclusion
The advancements in synthetic biological systems herald a new era for lactoferrin production. By unlocking the potential for large-scale, controlled production through genetic engineering, the doors to new applications in the food industry and pharmaceuticals are wide open. With continued research and innovation, the future looks bright for lactoferrin, promising a boost to health and well-being on a global scale.








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)