Revolutionary Breakthrough: How Earthquake-like Events Propel Cellular Movement!
2025-08-25
Author: Jacques
Unlocking the Secrets of Cell Mobility
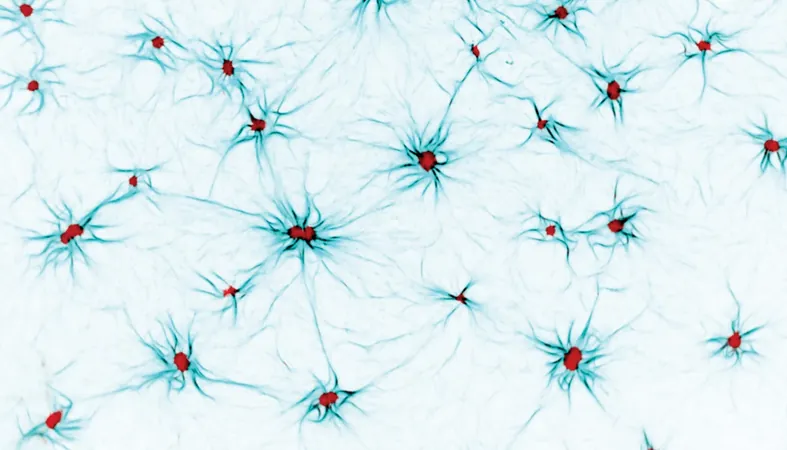
In a fascinating journey into the microscopic world, researchers have successfully recreated a bizarre, yet crucial component of animal cells—the actomyosin cortex. This innovative meshwork of structural and motor proteins not only defines cell shape but plays a pivotal role in cellular movement.
The Phenomenon of 'Cytoquakes'
During their groundbreaking experiments, scientists observed explosive energy bursts within artificial cytoskeletons that eerily mimic the violent energy release of earthquakes. These events, dubbed 'cytoquakes,' hint at a finely-tuned process where cells can dramatically reshape themselves by 'blowing up' their cytoskeletons.
A Closer Look at Self-Organized Criticality
These extraordinary bursts of energy arise in networks that possess a specific structural arrangement, leading to conclusions about self-organized criticality—a concept likened to the avalanche of sand slipping downhill or the release of pent-up tension during an earthquake.
Despite skepticism surrounding self-organized criticality in broader contexts, this study could provide a vital microscopic perspective, shedding light on how dynamic systems operate at a cellular level.
Decoding the Complex Actomyosin Cortex
For decades, the actomyosin cortex has left scientists scratching their heads. Once merely viewed as a gelatinous mass, it’s now recognized as a versatile network of stiff, yet flexible, filaments that undergoes constant rebuilding every 30 seconds, even while a cell appears to be idle.
Inside the Cellular Engine: A New Approach
Previous attempts to understand this structure involved external manipulations, but now researchers took a fresh approach by recreating the intracellular machinery. Senior author Michael Murrell, a physicist at Yale University, led the charge in engineering a model that accurately simulates the dynamic behaviors of living cells.
In a controlled environment using specialized glass slides, they combined actin monomers with precise catalysts to develop branched networks of filaments. Enhancements included adding fluorescent markers for tracking movements, providing fresh insights into the dance of cellular mechanics.
Exciting Discoveries: The Nature of Cytoquakes
What emerged were patterns of cytoquakes characteristic of self-organized criticality, but they only occurred in moderately branched actin networks. Murrell notes, 'There's a very specific organization in which this happens.' Too much tangling hinders the necessary myosin assembly needed for significant structural changes, while just the right amount of branching sets the stage for rapid dismantling and reconstruction of the cytoskeleton.
The Implications of a Dynamic Cytoskeleton
'It's easier to dismantle and rebuild than to try and reshape,' Murrell explains, emphasizing how this rapid accessibility benefits cells needing to mobilize. Noted physicist John C. Crocker praises the research as a game-changer, underscoring the meticulous nature of the biochemical approaches taken.
With this cutting-edge research, the mysteries of how cells navigate their environments are gradually unraveling, paving the way for future breakthroughs in cellular biology and medicine.








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)