Breakthrough Discovery Reveals How Damaged DNA Sparks Immune Response, Paving the Way for Enhanced Cancer Treatments!
2025-01-06
Author: Liam
Introduction
In an exciting development from the University of California, Irvine, researchers have unveiled a groundbreaking mechanism that activates an inflammatory immune response in cells with damaged DNA. This revelation not only enhances our comprehension of cell signaling but also opens new avenues for creating more effective cancer treatments.
Publication Details
Published today in the prestigious journal *Nature Structural & Molecular Biology*, the study highlights how exposure to UV radiation or certain chemotherapy agents triggers a unique response in severely damaged cells, effectively preventing their progression to cancerous states.
Research Insights
Lead researcher Rémé Buisson, an associate professor of biological chemistry at UC Irvine, expressed optimism regarding the implications of this research for cancer therapy. “This discovery could significantly transform how we approach cancer treatment. Understanding the nuanced reactions of different cancer cells to DNA damage could lead to highly personalized therapies, minimizing side effects and enhancing patients' quality of life,” he stated.
Historical Context and New Discoveries
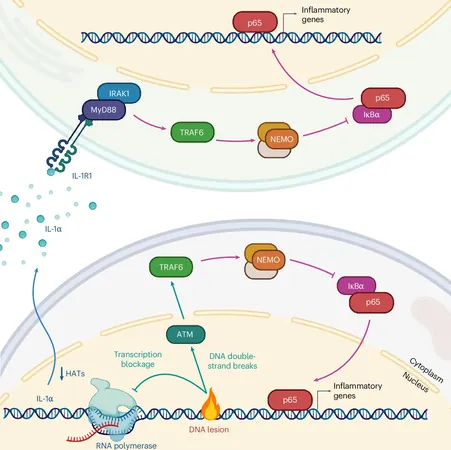
Historically, it was known that double-strand DNA breaks activate the ATM enzyme, which then initiates the NF-κB protein signaling cascade, resulting in inflammatory signal production. However, this new study, led by postdoctoral fellow Elodie Bournique with support from graduate student Ambrocio Sanchez, has shed light on an additional layer of complexity. It reveals that when cells sustain damage from UV light or chemotherapeutic agents like actinomycin D or camptothecin, the IRAK1 enzyme plays a crucial role in signaling for immune cell recruitment.
Methodology
Employing advanced imaging techniques, the team meticulously analyzed NF-κB regulation at the cellular level. Their discoveries unveiled how specific injuries lead to the release of the IL-1α protein, which, unlike other signaling molecules, does not act on the original cell but communicates with neighboring cells to activate the IRAK1 protein, thus prompting the NF-κB inflammatory response.
Implications for Personalized Medicine
“Our findings not only enhance our understanding of how various chemotherapeutic drugs induce DNA damage but also reveal that IL-1α and IRAK1 protein levels significantly differ across various cancer cell types,” Buisson explained. “This indicates a variability in patient reactions to treatment, emphasizing the need for personalized medical strategies.”
Future Research Directions
The researchers aim to further validate their findings through experiments on mouse models genetically modified to lack certain factors associated with this newly identified pathway. This step is crucial in translating laboratory discoveries into viable therapeutic interventions that could revolutionize cancer care.
Conclusion
Stay tuned as these innovative studies could change the landscape of cancer treatment as we know it, potentially leading to a future where therapies are customized to harness the immune system's power against tumors!







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)