Unlocking the Secrets of Water: Why Does Heavy Water Act So Much Like Its Lighter Cousin?
2025-04-09
Author: Rajesh
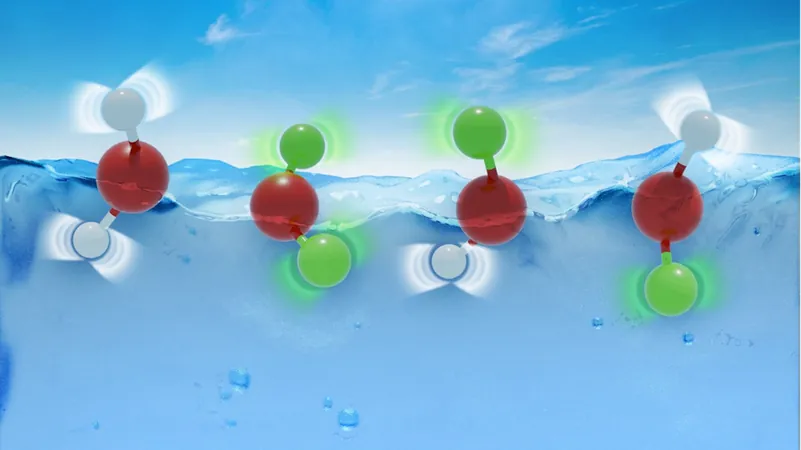
Did you know that over 70% of our planet is covered in water? But there’s an intriguing twist—hidden among the H2O, there's a rare form known as "heavy water." In heavy water, hydrogen atoms are swapped for their heavier counterparts, deuterium. This seemingly slight change has sparked a remarkable discovery about why heavy water and regular water behave so similarly!
At first glance, one might assume that the added weight of deuterium would cause heavy water to differ greatly from H2O. Astonishingly, their freezing points diverge by a mere 4°C! How can this be? Researchers from the Max Planck Institute for Polymer Research, led by Director Mischa Bonn, have cracked this enigma by examining the fascinating world of quantum mechanics.
The Quantum Dance of Atoms: Zero-Point Energy Unveiled
Even at absolute zero (-273°C), atoms aren't completely still; they maintain a subtle movement called "zero-point energy." In regular water, hydrogen atoms exist in a sort of 'cloud' around an average position, thanks to their low mass—this leads to pronounced vibrations.
The Intriguing Role of Deuterium
When hydrogen is replaced with the heftier deuterium, these atoms vibrate less vigorously, bringing them closer to the oxygen atom. This affects the spacing within the water molecule and increases the distance between neighboring molecules, diminishing what’s known as the binding energy—the energy required to separate molecules during processes like freezing.
The Counterbalancing Dance of Quantum Effects
But here’s where it gets even more fascinating! The deuterium atom can also oscillate in a direction that counteracts the changes imposed by its proximity to the oxygen atom, effectively balancing the reduced binding energy. These dual effects, one enhancing and the other diminishing molecular bonds, lead to only a slight difference in freezing temperatures.
A Groundbreaking Discovery in Quantum Physics
To unravel this complex relationship, researchers employed a cutting-edge technique called heterodyne-detected sum-frequency generation (HD-SFG) spectroscopy. This allowed them to dive deep into the properties of water’s surface layer, observing how molecules interact in ways never quantified before.
This groundbreaking study, now published in Science Advances, presents the first experimental evidence for the tug-of-war between intramolecular and intermolecular quantum effects in water—a phenomenon long theorized but never actually observed.
Understanding these quantum nuances is not just academic; it has vital implications spanning fields like climate science and biochemistry, where water's properties are crucial. With this insightful approach, researchers are opening new doors to explore quantum effects in other complex systems, potentially transforming our comprehension of matter itself.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)