Revolutionary Bimetallene Catalyst Transforms Biomass into Green Fuels Efficiently!
2025-04-08
Author: Sarah
Introduction
In a remarkable technological leap, researchers have unveiled a new bimetallene catalyst that paves the way for sustainable energy production from biomass, a vital component of the emerging bioeconomy.
Significance of HMF
At the heart of this innovation is 5-hydroxymethylfurfural (HMF), a key compound derived from lignocellulosic biomass, which has the potential to replace traditional petroleum-based chemical building blocks.
Selective Hydrogenation Challenges
The selective hydrogenation of HMF to form 2,5-dihydroxymethylfuran (DHMF) is particularly significant due to its importance as a precursor in pharmaceuticals, nucleoside analogs, and specialty polymers. However, existing thermocatalytic methods for HMF hydrogenation often require extreme conditions—high temperatures and pressures—that lead to considerable energy consumption and complexities in process scaling.
Electrochemical Hydrogenation Innovation
Now, a game-changing study recently published in the Chinese Journal of Catalysis highlights a groundbreaking method: electrochemical hydrogenation (ECH) carried out under ambient conditions.
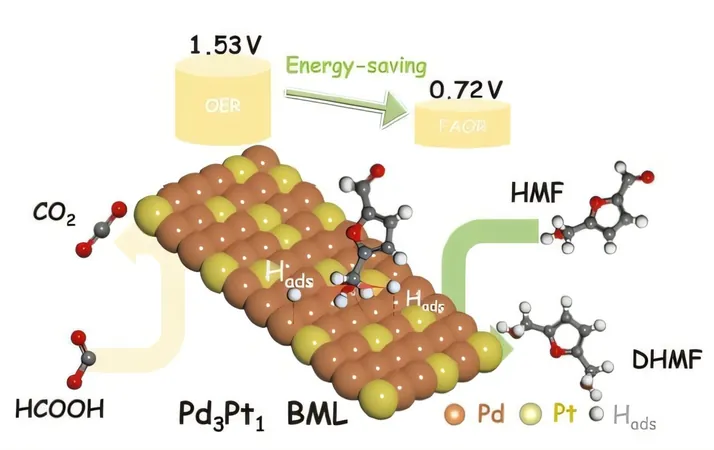
Introduction of Pd3Pt1 Catalyst
Spearheaded by Professor Yu Chen and his team at Shaanxi Normal University in China, this research introduces an innovative bimetallene catalyst, namely Pd3Pt1, which significantly enhances the energy efficiency of this process.
Synthesis and Performance
The Pd3Pt1 bimetallene catalyst is synthesized through a straightforward galvanic displacement reaction, showcasing a unique two-dimensional metallic structure.
Its atomically dispersed platinum within the palladium matrix boosts catalytic advantages: the catalyst achieves a remarkable Faradaic efficiency exceeding 93% and maintains a DHMF selectivity above 66% under mild operational conditions.
Unique Catalyst Features
What makes this catalyst stand out? The synergy of geometric and electronic effects plays a crucial role in its exceptional performance.
Advancements in Electrochemical Systems
Additionally, a critical advancement in this research is the pairing of HMF electrochemical hydrogenation at the cathode with formic acid oxidation at the anode—a strategic coupling that bypasses the commonly slow oxygen evolution reaction (OER) that usually hampers the efficiency of electrochemical systems.
Performance Metrics
The innovative Pd3Pt1 BML||Pd3Pt1 BML electrolyzer achieves an impressive current density of 10 mA cm-2 at an ultra-low cell voltage of just 0.72 V for HMF electrochemical hydrogenation.
Conclusion
In conclusion, this groundbreaking catalyst not only offers a cleaner and more sustainable pathway to biofuel production but also sets the stage for a future where renewable resources play a dominant role in meeting global energy demands.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)