Unlocking the Secrets of Protein Folding: A Study Unveils Core Packing Fractions
2025-03-28
Author: Li
Unlocking the Secrets of Protein Folding: A Study Unveils Core Packing Fractions
In the intricate world of biology, proteins—complex polymers made of dozens to hundreds of amino acids—play essential roles such as catalyzing chemical reactions, transporting molecules, and repairing DNA. The correct functionality of these proteins hinges on their ability to fold into precise three-dimensional shapes, a delicate and vital process for life itself.
Despite significant advancements in our understanding of protein folding, several mysteries remain unsolved. A groundbreaking study published in PRX Life has offered new insights into this phenomenon, paving the way for innovative protein designs geared towards drug development, cutting-edge biomaterials, and beyond.
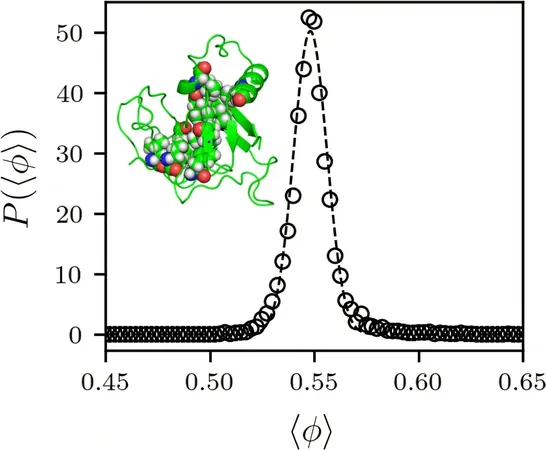
Under the guidance of Corey O'Hern, a prominent professor specializing in mechanical engineering and materials science, researchers employed advanced computational models to analyze all globular proteins stored in the Protein Data Bank—a publicly accessible online repository of protein structures. They meticulously measured the interior core regions of these proteins to assess their density, discovering that an intriguing trend emerged: all proteins possess a core packing fraction of 55%. This means that 55% of the core volume is occupied by atoms.
This discovery raised pivotal questions for the research team. “Why does every protein share the same packing fraction? More specifically, what makes that value 55%?” O'Hern explained that the packing fraction ceases to increase when proteins “jam” or become rigid, preventing the amino acids from compressing further during folding. The rate at which particles, including amino acids, can tightly pack together is heavily influenced by their shapes. For instance, spherical particles achieve maximum packing at around 64%, but the majority of amino acids are not spherical in nature.
The complexity of amino acids—many being elongated and rough thanks to side chains and bonded hydrogen atoms—contributes to this lower packing fraction. O'Hern elaborated that the physics of soft matter indicates that jammed configurations of these complex, bumpy particles typically result in less dense packings compared to their smooth, spherical counterparts.
A fascinating area for future inquiry is whether the packing fraction of protein cores can exceed 55%, especially beyond standard physiological conditions. Previous studies have demonstrated that proteins subjected to high pressures, similar to those found in deep-sea hydrothermal vents—sites possibly linked to the origin of life—exhibit core packing fractions between 58% and 60%.
"Understanding the properties of protein cores during typical folding scenarios opens new avenues," stated Alex Grigas, a Ph.D. candidate and lead author of the study. "With changes in solvent conditions, pressure, or temperature, there may be opportunities to enhance the packing efficiency of amino acids."
With this innovative research, the realm of protein design is on the cusp of transformation. Instead of merely altering the sequences of amino acids for new protein structures and functions, the newfound understanding of packing fractions could integrate a broader range of variables to design proteins with enhanced properties. This study not only advances our comprehension of protein folding but may also shed light on the very origins of life on Earth, propelling the scientific community into exciting new territories of exploration.
Stay tuned as scientists unveil more mysteries surrounding proteins, potentially revolutionizing fields such as medicine and synthetic biology!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)