Unlocking the Secrets of Alzheimer's: How Different APOE Proteins Influence Brain Health
2025-05-27
Author: Nur
Revolutionary Study Sheds Light on APOE Proteins and Alzheimer's Disease
A groundbreaking study published in *Nature Communications* today reveals intriguing insights into how different versions of the APOE protein impact microglial function in the context of Alzheimer's disease. Spearheaded by Dr. Sarah Marzi and Dr. Kitty Murphy at the UK Dementia Research Institute, King's College London, this research paves the way for personalized interventions based on individual APOE genotypes.
The Alzheimer's Crisis: A Race Against Time
Alzheimer's disease, the leading cause of dementia in the UK, strikes 1 in 14 individuals over the age of 65, presenting a staggering challenge to public health. The disease is marked by dangerous buildups of amyloid plaques and tau tangles within the brain, impairing cognitive function and quality of life.
The APOE Mystery: Unpacking the Genetic Puzzle
The apolipoprotein E (APOE) gene stands out as a significant genetic risk factor for Alzheimer’s. It comes in three forms: APOE2, APOE3, and APOE4, where APOE4 heightens the risk of developing Alzheimer’s, while APOE2 is linked to a lower risk. However, the mechanisms driving these stark differences remain largely elusive.
A Groundbreaking Xenotransplantation Model!
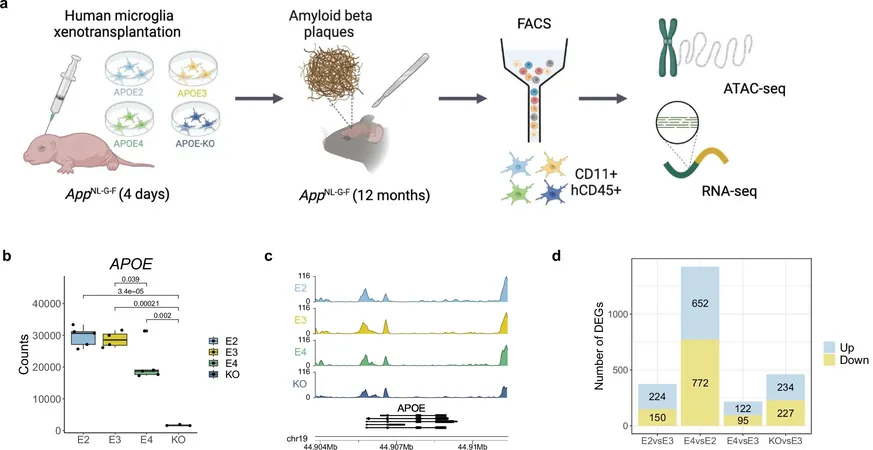
In this innovative study, researchers made a major leap by examining how these APOE isoforms affect microglia—the brain's immune cells—within a unique human 'xenotransplantation model.' Here, human microglia were cultivated from stem cells, engineered to express specific APOE versions, and then transplanted into the brains of mice with amyloid plaques.
Profound Changes Unearthed in Microglial Function
The study unveiled remarkable alterations in both the transcriptomic profiles and chromatin accessibility of microglia, based on the specific APOE isoform expressed. The most striking differences emerged in microglia expressing APOE2 compared to those expressing APOE4.
Microglia with the APOE4 variant demonstrated heightened production of cytokines—key signaling molecules in immune regulation—but showed diminished movement and a decreased ability to adopt protective roles. Their phagocytic capacity, essential for clearing away debris and pathogens, also appeared compromised.
On the flip side, APOE2 microglia exhibited enhanced gene expression linked to proliferation and migration, alongside a significantly reduced inflammatory response. Notably, these cells also displayed increased DNA binding of the vitamin D receptor, hinting at a potential link between vitamin D levels and Alzheimer's risk.
A New Frontier in Alzheimer's Research
This study highlights that microglial responses to amyloid pathology vastly differ based on the APOE isoform. It emphasizes the crucial need to consider genetic influences alongside microglial states in understanding the progression of Alzheimer's disease.
Dr. Sarah Marzi, a Senior Lecturer in Neuroscience and the study's lead author, stated, "Our findings stress the intricate interplay of genetic, epigenetic, and environmental factors shaping microglial responses in Alzheimer's. The differences we observed in microglia expressing various isoforms of the APOE gene are truly remarkable."
What’s Next? The Therapeutic Potential Awaits!
With the implications of these findings, researchers are set to explore new therapeutic avenues targeting microglial responses tailored to specific APOE genotypes. The future of Alzheimer's treatment might just be rooted in understanding these genetic intricacies.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)