The Alarming Rise of Antibiotic Resistance in Mycoplasma pneumoniae Among Children in Russian Hospitals: Insights from Recent Studies
2025-03-15
Author: Wei
Mycoplasma pneumoniae (MP) is a tiny, self-replicating bacterium that lacks a conventional cell wall, posing a significant threat as it can lead to severe respiratory infections in both children and adults. This bacterium spreads through airborne droplets, usually in crowded places such as homes and schools, making it particularly problematic during peak seasons. Symptoms of infection are diverse, ranging from mild upper respiratory complaints to atypical pneumonia, primarily affecting children and adolescents. The incidence of MP infections typically spikes during the colder months, between summer and early spring, driven by factors like temperature and humidity.
Recent data indicates a resurgence in Mycoplasma pneumoniae cases following a lull during the COVID-19 pandemic. In fall 2023, hospitals in Russia reported an increase in infections, raising concerns about potential co-infections with other viral pathogens. Research indicates that such co-infections can exacerbate the severity of Mycoplasma pneumoniae-related respiratory diseases.
As the treatment landscape evolves, resistance to antibiotics like macrolides, traditionally employed for MP infections, has become a growing concern. The current first-line treatment options face challenges due to emerging resistant strains. In recent analyses, mutations linked to macrolide resistance have been identified in 40% of tested samples, raising alarms over antibiotic efficacy in treating these infections.
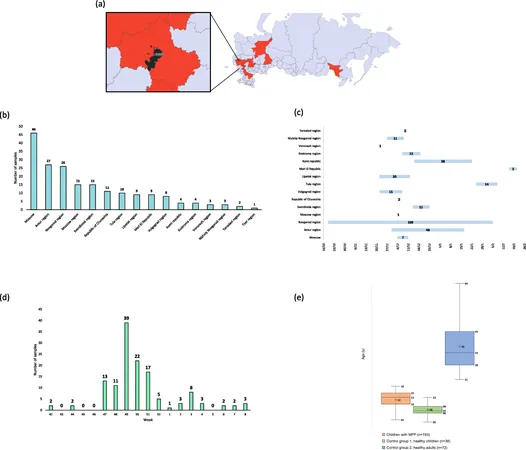
Our study involved a thorough examination of 193 hospitalized children diagnosed with pneumonia caused by Mycoplasma pneumoniae between October 2023 and February 2024 across various regions in Russia. We confirmed MP infections through clinical assessments, imaging studies, and PCR testing.
To better understand the broader context, we compared viral infections present in these pediatric patients with healthy control groups, revealing a staggering 62% co-infection rate among those diagnosed with MP pneumonia. The most frequently detected viral pathogens were human parainfluenza viruses types 3 and 4, along with SARS-CoV-2, corroborating findings of other studies that suggest viral co-infections significantly impact disease severity.
Demographic analysis showed that the majority of affected children were school-aged, predominantly between 7 and 17 years. Interestingly, while some healthy individuals also tested positive for Mycoplasma pneumoniae, they exhibited no respiratory symptoms, suggesting they are asymptomatic carriers. This highlights the challenge in diagnosing and treating infections accurately, as current testing methods may fail to differentiate between active infections and simple colonization.
Furthermore, the study delves into genetic mutations associated with antibiotic resistance. Of particular note was the discovery of mutations in the 23S rRNA gene responsible for macrolide resistance, which raises the pressing question of how such resistance trends might evolve in the face of rising MP infections.
The investigation also included the genome characterization of Mycoplasma pneumoniae isolates from Russian patients, marking a critical step in understanding the genetic diversity and resistance mechanisms in this pathogen. Complete genome sequencing endeavors revealed a significant level of similarity with strains found in southeastern Asia, suggesting possible links and sharing of resistance traits across regions.
As researchers continue to track the evolution of antibiotic resistance and analyze the implications of viral co-infections in Mycoplasma pneumoniae infections, it becomes increasingly clear that a coordinated public health response is essential to mitigate these threats. Protecting children's health requires not just effective treatment protocols but also a comprehensive understanding of the epidemiological landscape surrounding respiratory infections, especially in complex viral and bacterial ecosystems.
The findings underscore an urgent need for enhanced surveillance, rapid diagnostics, and the development of new therapeutic strategies to combat rising antibiotic resistance and ensure better health outcomes for affected populations.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)