Shocking Discovery: Forever Chemicals More Acidic Than Ever Imagined!
2025-09-04
Author: Daniel
The Alarming Truth About PFAS Acidity
BUFFALO, N.Y. — A groundbreaking study from the University at Buffalo reveals that per- and polyfluoroalkyl substances (PFAS), known as "forever chemicals," are far more acidic than scientists initially believed. This astonishing finding could change how we understand their environmental impact and potential risks to human health.
What Makes PFAS So Dangerous?
PFAS are notorious for their resilience in the environment, largely due to their highly acidic nature. This acidity allows them to easily release protons and become negatively charged, a characteristic that facilitates their dissolution and spread in water.
Revolutionary Research Methods
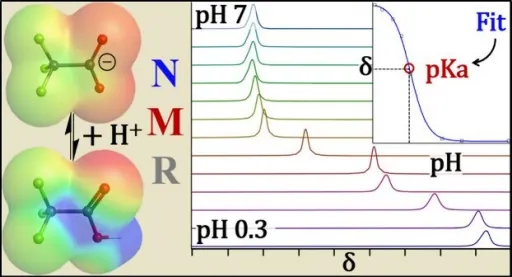
The team at the University at Buffalo employed a new experimental technique to measure the acidity of ten types of PFAS and their common breakdown products. Their findings, published in Environmental Science & Technology Letters, showed much lower acid dissociation constants (pKa) than previous studies had indicated. For instance, the pKa for GenX—a Teflon substitute—was revealed to be approximately a staggering one thousand times lower than earlier estimates.
Understanding pKa: Why It Matters
The lower the pKa, the more readily a chemical can release a proton and adopt a charged state. As Dr. Alexander Hoepker, the study's lead author, explains, this means earlier perceptions of PFAS acidity and behavior in the environment may have been significantly skewed.
Innovative Techniques to Improve Accuracy
Historically, pKa measurements for PFAS have varied greatly among scientists, creating confusion about their environmental behavior. One significant issue was that PFAS tend to stick to glass, impacting traditional measurement methods. To overcome this, the researchers used a cutting-edge technique called nuclear magnetic resonance (NMR) spectroscopy.
This method allows them to detect atom-level changes in PFAS molecules, providing a clearer picture of their charged or neutral states, which older methods could not accomplish.
Surprising Findings on PFOA and New PFAS
Remarkably, the team uncovered a pKa of -0.27 for PFOA, an alarming value suggesting it remains negatively charged at nearly any environmental pH. This stands in stark contrast to previous estimates that varied widely from 1 to 3.8.
The research also unveiled new information about trifluoroacetic acid (TFA), identified as a rapidly mobilizing PFAS, with a pKa around 0.03—much more acidic than earlier assessments.
Implications for the Future
These revelations have far-reaching consequences. They pave the way for future research, helping scientists validate computational models, develop machine learning techniques for predicting pKa values of new PFAS, and ultimately refine methods for analyzing and remediating these hazardous substances.
As Dr. Diana Aga, director of the RENEW Institute, notes, having accurate pKa measurements is essential for understanding how these chemicals spread, which will enhance risk assessment strategies and foster better environmental protections.
A Step Forward for Environmental Science
With significant contributions from researchers in both the U.S. and Spain, this study stands as a pivotal moment in the ongoing battle against PFAS pollution, providing critical insights that could lead to more effective management and remediation techniques.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)