Shocking Discoveries: How Macrophage Ferroptosis Fuels Pulmonary Venous Disease
2025-09-22
Author: Nur
Understanding the Link Between Macrophages and Pulmonary Venous Disease
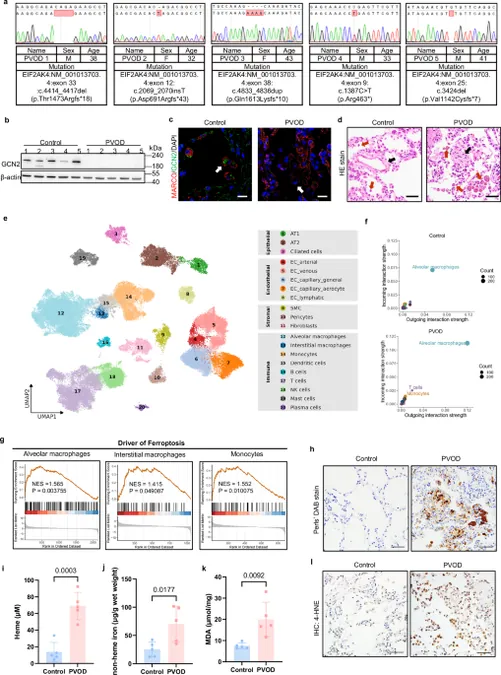
Recent research has unveiled that genetic mutations in the EIF2AK4 gene significantly impact lung health, particularly in patients with pulmonary veno-occlusive disease (PVOD). Analysis reveals that levels of ferroptosis-related gene expression are notably heightened in lung macrophages of these patients, prompting scientists to investigate how these mutations influence the disease's development.
A Closer Look at EIF2AK4 and Its Macrophage Impact
Through immunoblotting and immunostaining, researchers assessed GCN2, the protein encoded by EIF2AK4, revealing a complete absence of this protein in PVOD patients. In stark contrast, healthy individuals showcased robust GCN2 expression in their lung macrophages. This difference suggests that macrophages may play a pivotal role in the disease's pathophysiology.
Breaking Down Ferroptosis in Lung Macrophages
Single-cell RNA sequencing demonstrated that in PVOD patients, macrophages exhibited markedly enhanced ferroptosis-associated gene expression. Elevated iron levels were also discovered, which align with the pathology of PVOD—characterized by pulmonary hemorrhage and iron accumulation. This critical discovery suggests that ferroptosis could be a leading factor driving the disease.
Innovation in Animal Models for PVOD Research
To further understand these mechanisms, researchers developed a mouse model with the same EIF2AK4 mutation. When subjected to hypoxia, these mice exhibited clear signs of pulmonary vascular dysfunction, signaling a dire need for innovative treatment approaches.
Hope on the Horizon: Ferroptosis Inhibition Shows Promise
Remarkably, the ferroptosis inhibitor Ferrostatin-1 (Fer-1) demonstrated potential in reversing PVOD-like symptoms in these mice. Treatment with Fer-1 led to significant drops in pulmonary vascular resistance, opening doors for new therapeutic strategies.
Exploring Macrophage Ferroptosis in Other Models
In addition to genetic PVOD models, researchers also evaluated non-genetic forms of the disease. Insights from rat models exposed to MMC (a chemotherapy agent) revealed heightened macrophage ferroptosis, further confirming that this pathological mechanism is prevalent across different types of PVOD.
Final Thoughts: A Complex Dance of Genetics and Environment
The findings underscore the intricate interplay between genetics and environmental stressors in the development of PVOD. As macrophages become increasingly susceptible to ferroptosis due to GCN2 deficiency and iron accumulation, addressing these pathways presents a critical avenue for therapeutic development. The implications of this research could alter the landscape of treatment strategies for PVOD.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)