Revolutionizing Mobility: Insights from the SARA-OBS Trial on Sarcopenia in Seniors
2025-08-04
Author: Mei
Unveiling the SARA-OBS Study: A Bold Step in Geriatric Healthcare
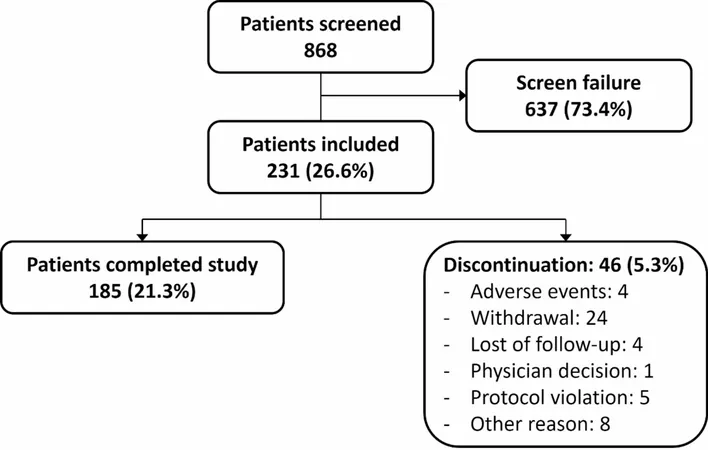
The SARA-OBS trial—an ambitious 6-month observational study—took a deep dive into the lives of older adults, aged 65 and above, grappling with sarcopenia and associated obesity. Conducted across 11 centers in Belgium, France, Italy, and the USA, the study aimed to shed light on how these conditions impact mobility risks. Participants were enrolled from February 2017 to October 2018, with meticulous recruitment processes ensuring only the eligible were selected.
Defining Eligibility: Who Made the Cut?
To join the SARA-OBS trial, participants had to meet stringent criteria, including: - Being 65 or older and living independently. - Scoring 8 or less on the Short Physical Performance Battery (SPPB), indicating potential mobility issues. - Failing to meet specific body composition benchmarks defined by the FNIH guidelines. While various medications were permitted, anabolic drugs like testosterone were off-limits, emphasizing the trial's focus on natural treatment pathways.
Measuring Success: Primary and Secondary Outcomes
The heart of the study lay in its outcomes. The primary endpoint focused on changes in gait speed, assessed with the 400 m Walking Test (400MWT). But that wasn’t all; secondary endpoints included muscle strength as measured by handgrip performance, distance covered in a 6-minute walk (6MWD), and several quality of life assessments via the Sarcopenia and Quality of Life (SarQoL) questionnaire.
Promising Findings: Initial Results
As researchers delved into the data, they discovered intriguing trends. The overall gait speed showed a slight decline, hinting at possible deterioration over the follow-up period. Interestingly, participants with a baseline gait speed of less than 0.8 m/s exhibited significant changes, raising alarms about their mobility and functional capabilities.
Uncovering the Biomarkers: A New Frontier
Adding a layer of complexity, the trial investigated biomarkers such as myostatin and inflammatory indicators like IL-6 and hsCRP. These markers could serve as crucial tools in understanding sarcopenia and its implications for mobility, though no significant statistical changes were reported.
Facing the Challenges: Safety and Participant Well-being
Safety was paramount in the SARA-OBS trial, with 32.4% of participants reporting at least one adverse event—mostly falls, a critical concern in this vulnerable population. Overall, there were no fatalities, but participants dealt with various health issues ranging from mild to severe.
The Bigger Picture: Impacts on Future Research
The SARA-OBS trial opens doors to meaningful discussions about sarcopenia in the elderly, laying the groundwork for future research and potential interventions targeting this demographic. The insights gleaned could lead to more effective strategies for enhancing mobility and overall quality of life in seniors at risk of mobility disability.
Conclusion: Moving Forward with Purpose
As the landscape of geriatric healthcare evolves, the findings of SARA-OBS highlight the urgent need to address sarcopenia and its related challenges. With careful analysis and targeted future studies, there’s hope for innovative treatments that could revolutionize the way we support our aging population.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)