Revolutionary Technique Developed to Correct Protein Misplacement in Cells—Could This Cure Cancer?
2024-09-21
Revolutionary Technique Developed to Correct Protein Misplacement in Cells—Could This Cure Cancer?
Scientists at Stanford University have made a groundbreaking discovery that could change the way we approach diseases tied to protein misplacement, including various cancers and neurodegenerative disorders. Let's delve into how this innovation works and its potential implications for medicine.
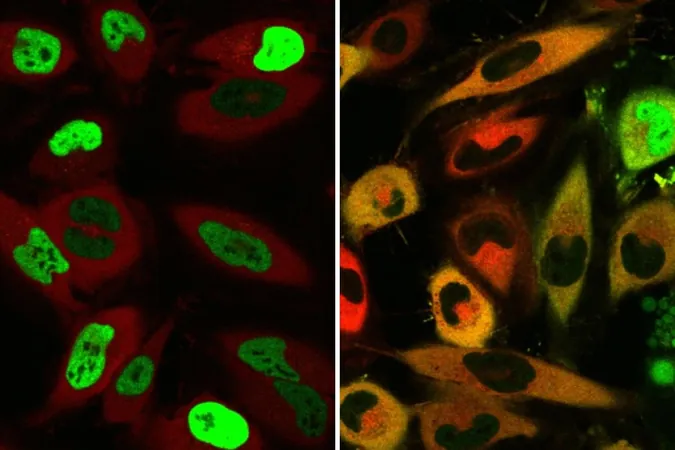
Cells are like bustling cities that require proteins to be in specific locations to maintain order and function. Misplaced proteins can disrupt these operations, leading to serious health complications. For instance, in certain cancers, vital proteins that monitor DNA replication may wander far from their intended locations in the nucleus, contributing to uncontrolled cancer growth.
A research team led by Steven Banik, an assistant professor of chemistry at Stanford and a scholar at Sarafan ChEM-H, has developed a novel approach to redirect these misplaced proteins back to their rightful places. They have introduced a unique class of molecules termed "targeted relocalization activating molecules" (TRAMs). These TRAMs essentially act as smart delivery vehicles, re-engineering natural shuttles within the cell to transport proteins that have been misallocated.
Banik emphasizes, "We are taking proteins that are lost and bringing them back home." This revelation could lead to powerful therapies aimed at correcting the protein misplacement associated with various diseases, as well as augmenting cellular functions.
Understanding the Mechanism
Cells are compartmentalized into various regions such as the nucleus and mitochondria, where proteins perform critical actions like energy production and gene regulation. However, the complex environment of a cell presents challenges—proteins must navigate through a dense matrix of other cellular components to reach their destinations.
Proteins that become misplaced often result in severe consequences. For example, in amyotrophic lateral sclerosis (ALS), the FUS protein is redirected from the nucleus to the cytoplasm, where it can form toxic aggregates that damage cells. To combat these malfunctions, Banik's team devised a solution that employs naturally occurring shuttles to carry proteins back to their intended locations.
Their strategy involves creating TRAMs that have dual functionalities: one end binds to the shuttle, while the other attaches to the misplaced protein, facilitating its transport back to the nucleus or other required regions.
Success in Trials
Banik’s graduate student, Christine Ng, has played an integral role in testing this method. After designing TRAMs to move proteins in and out of the nucleus, she developed a new technique to quantify protein localization in individual cells. "Nature is inherently complex, so interdisciplinary approaches are crucial," Ng notes, highlighting the importance of blending knowledge from diverse fields to tackle scientific challenges.
Through rigorous experimentation, the team found that their TRAMs could successfully relocate the problematic FUS protein back to the nucleus, thus reducing the formation of toxic clumps and enhancing cell viability.
The team also explored the potential of creating TRAMs that mimic mutations known to confer resistance to neurodegeneration. Their results were promising, demonstrating that these TRAMs could carry proteins down the axon, enhancing cellular resistance to stress related to neurodegenerative conditions.
Looking Ahead
Despite the success achieved with these initial targets, challenges remain. Identifying the optimal passenger-targeting components for TRAMs is a complex task, as scientists have yet to discover all possible binding molecules. For now, the team is applying genetic engineering techniques to tag proteins, creating a pathway for future research.
Moreover, the versatility of TRAMs extends beyond remedying protein misplacement. They could potentially unlock new functionalities within cells by delivering healthy proteins to new locations, thus broadening our understanding of cellular biology.
As Banik aptly states, "We are just starting to learn the rules. If we shift the balance, what new functions could we unlock?" This pioneering work not only signals hope for therapeutic advancements but also opens exciting avenues for future research in cellular biology and medicine.
Stay tuned as this revolutionary technique unfolds its potential in the fight against diseases like cancer and ALS!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)