Revolutionary Stem Cell Research Unlocks Secrets of Kidney Development: Are Artificial Kidneys on the Horizon?
2024-10-01
Introduction
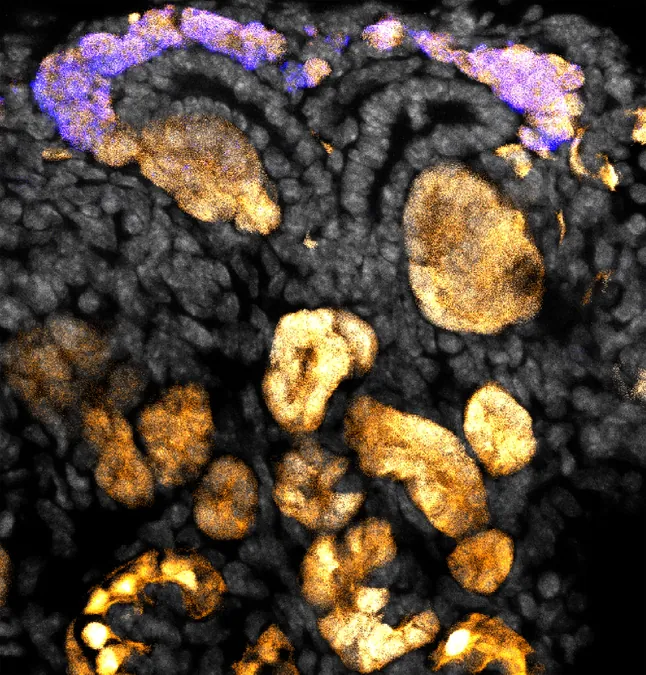
Scientists from the USC Stem Cell community, led by renowned expert Andy McMahon, have made groundbreaking strides in understanding how progenitor cells self-renew, differentiate, and ultimately form the foundational structures of early kidney development. Their recent studies, published in the prestigious journal Development, shed light on the pivotal Wnt signaling pathway—a network of essential molecules integral to multicellular life and a subject of research for over four decades that influences both development and various diseases.
Importance of Nephron Progenitor Cells
The focus of these studies is the nephron progenitor cells (NPCs), which are responsible for the formation of nephrons—the functional filtering units of the kidney. According to Helena Bugacov, a primary author and Ph.D. graduate from McMahon’s lab now pursuing a medical degree, these crucial NPCs are absent by the time humans are born. Their disappearance means that postnatal kidneys cannot regenerate new nephrons, a situation leading to the dire need for kidney transplants as nephron function declines. Unfortunately, the availability of donor kidneys is insufficient to meet demand.
Research Findings on Wnt Signaling
So, why does this research matter? The insights derived from understanding Wnt signaling could propel efforts to create stem cell-derived artificial kidneys, offering a groundbreaking solution for those suffering from kidney diseases.
In the lab, the USC researchers cultivated NPCs and exposed them to varying concentrations of a chemical called CHIR, known to modulate the Wnt signaling pathway. Their work provided compelling evidence that low levels of Wnt signaling support the self-renewal of NPCs, ensuring the production of the 14,000 nephrons necessary for a mouse kidney. Conversely, elevated Wnt signaling stimulates the differentiation of these progenitor cells into mature kidney cells.
Key Discoveries on Nephron Formation
One of the pivotal findings showed that enhanced Wnt signaling facilitates the formation of renal vesicles, clusters that are precursors to nephrons. This crucial cellular transition is driven by beta-catenin, a critical player in cell adhesion, which binds NPCs together. Through a process known as mesenchymal-epithelial transition, these formerly mobile cells organize into a compact arrangement necessary for nephron formation.
Implications Beyond the Kidney
In further investigations, postdoctoral researcher Bálint Dér and Bugacov revealed how Wnt signaling orchestrates NPC aggregation, highlighting the transformation of loosely connected progenitor cells into a cohesive structure that precedes nephron development. This insight could not only redefine kidney research but also illuminate similar mechanisms in other organs, aiding broader medical applications.
Importantly, Dér shared that the implications of their discoveries extend beyond the kidney, emphasizing the Wnt pathway's influence across multiple organ systems—potentially impacting strategies for regenerative medicine and the treatment of various diseases.
Conclusion and Future Directions
Bugacov reflected on her experience in the McMahon Lab, praising the synthesis of developmental biology, stem cell science, and genetic engineering, all aimed at improving treatment options for individuals facing kidney ailments. With the ambitious goal of translating their findings into real-world solutions, the team hopes to pave the way for innovative treatments in kidney regeneration.
As the landscape of kidney disease treatment shifts, the prospect of developing stem cell-derived kidneys from progenitor cells ignites hope for millions waiting for transplants. The ongoing research at USC stands as a beacon of possibility, suggesting that a future without kidney shortages could soon become a reality.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)