Revolutionary RNA Biosensor Illuminates Upon Ligand Interaction!
2024-12-26
Author: Wei Ling
Introduction
In a groundbreaking advancement, researchers have successfully developed an RNA biosensor that emits fluorescence upon reacting with small inorganic metabolites—achieved not through the engineering of RNA itself, but by innovating the fluorogenic ligand molecule associated with it.
Background
The concept isn't entirely new—back in 2011, scientists pioneered the design of fluorescent RNA molecules that included an RNA aptamer binding to a hydroxybenzylidene imidazolinone (HBI) ligand. The fascinating twist is that neither the RNA aptamer nor the HBI ligand fluoresces independently. However, when they bind together, the combined structure lights up, allowing researchers to visualize RNA molecules within living cells.
Development Challenges
In a quest to enhance biosensor capabilities, researchers experimented with riboswitches—RNA components that alter their structure upon binding to specific molecules—integrating these with aptamers. The goal? To engineer sensors that would glow only in the presence of both an HBI and certain biomolecules, like S-adenosylmethionine. Yet, as chemist Enver Cagri Izgu from Rutgers University explains, devising such sensors has proven challenging, particularly for inorganic molecules that typically do not interact with RNA effectively. "RNA has a folding issue, especially in cellular environments," he notes. "This makes it incredibly hard to manipulate for biomedical applications."
A New Approach
In light of these challenges, Izgu proposed a different approach: "Why not create an organic molecule that interacts directly with the target metabolite?" His recent publication in *Angewandte Chemie* reveals the development of several preligands designed to generate a fluorogenic HBI upon the introduction of inorganic redox-signaling molecules, marking a key step in the evolution of biosensing aptamers.
Introducing Selectivity
To enhance functionality, the researchers introduced a caging group into the HBI structure, which is responsive to hydrogen peroxide while avoiding interactions with other reactive oxygen species. Remarkably, this preligand remains non-fluorescent until it is cleaved, demonstrating impressive selectivity. The team repeated this process with another functional group that reacts specifically with hydrogen sulfide yet remains inert to other reactive thiols, resulting in a similar transformation to HBI when interacting with its analyte.
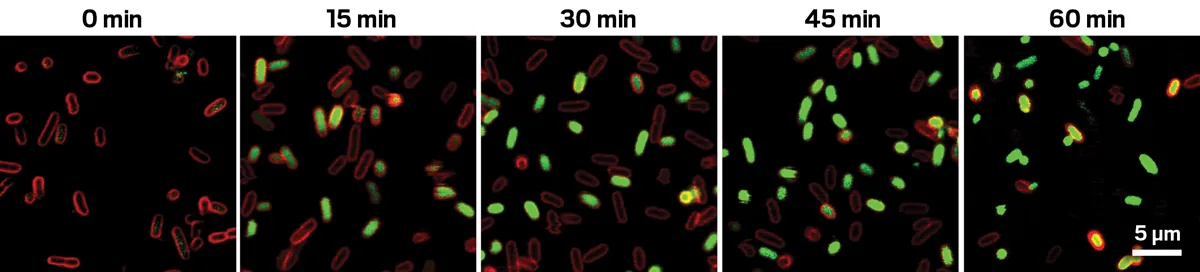
Experimental Outcomes
In experimental settings, when the researchers introduced the first preligand to bacteria engineered to express the aptamer, they observed a striking fluorescence when hydrogen peroxide was introduced. The cells illuminated, providing a promising technique for real-time monitoring of various biological processes.
Implications for Biomedicine
As these pioneering efforts continue, the implications for biomedicine are vast, potentially offering revolutionary tools for cellular imaging, disease detection, and beyond. Stay tuned for more updates on how this cutting-edge technology might change the landscape of biological research!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)