Revolutionary Mirror-Image Molecules Strike Back Against Superbugs!
2025-05-28
Author: Arjun
Antimicrobial resistance (AMR) has stealthily emerged as a formidable foe in our battle against bacterial infections since penicillin was discovered nearly a century ago. This crisis claims a staggering 1.27 million lives annually and significantly contributed to almost five million deaths in 2019 alone, placing antibiotic-resistant infections above HIV and malaria on the list of global health threats.
In a groundbreaking effort to tackle this pressing issue, a dynamic team from four schools at the University of Pennsylvania, spearheaded by Hydar Ali and César de la Fuente, has discovered an innovative method for treating infections.
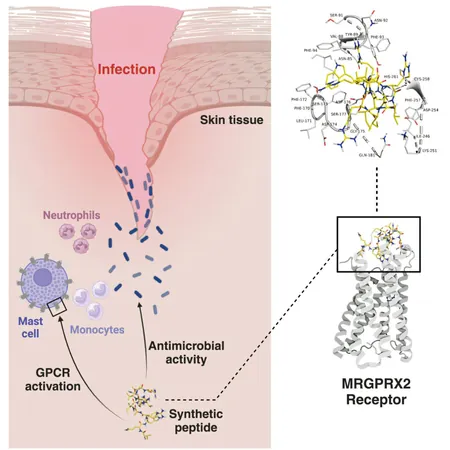
Their pivotal findings, published in the journal Cell Biomaterials, reveal a selection of short, positively charged peptides capable of not just piercing bacterial membranes but also rallying the body's immune response.
According to de la Fuente, a Presidential Associate Professor at the Perelman School of Medicine, "The winning candidates are constructed from D-amino acids, which are mirror images of the L-amino acids utilized by our bodies. This swap allows the peptides to evade the body's digestive enzymes, enabling them to remain intact and effective for much longer than previous peptide drugs."
In laboratory settings, these pioneer peptides successfully broke through the protective membranes of an especially resilient group of pathogens known as the ESKAPEEs—comprising notorious bacteria such as Enterococcus faecium, Staphylococcus aureus, and Escherichia coli.
Additionally, the peptides effectively engaged mast cells, the body's immune sentinels often associated with allergies. This activation prompts the release of inflammatory signals like TNF-α, IL-8, and CCL3, which call forth bacteria-fighting white blood cells to the scene.
Central to this remarkable one-two punch—penetrating bacterial defenses while simultaneously activating mast cells—is a cellular receptor named MRGPRX2. This receptor, abundant on skin-resident mast cells that protect against infections such as Staph, closely resembles a "panic button." When the designer peptide binds to it, the receptor sends out a distress signal.
This alert initiates a cascade of immune responses, including the release of histamine and a cocktail of cytokines and chemokines that attract neutrophils and monocytes to swarm and engulf the invading bacteria, while also coordinating long-term immune defenses.
Marcelo der Torossian Torres, a co-first author and research associate in de la Fuente's Machine Biology Group, explains, "By activating MRGPRX2, the peptide transforms the host's immune forces into allies. This unbeatable combo is unlike any traditional antibiotic."
In tests on living tissue, a single low-dose injection into MRSA-infected skin dramatically decreased bacterial numbers and accelerated healing. However, this benefit diminished in mice lacking MRGPRX2, highlighting the importance of immune activation for therapeutic success.
These mirror-image peptides present a dual threat—they attack microbes directly while also supercharging the immune response—requiring bacteria to overcome two significant challenges simultaneously, potentially slowing resistance.
As antibiotic resistance escalates globally, therapies that annihilate bacteria while boosting immunity represent a crucial new frontier. Preclinical studies are ongoing to further validate the effectiveness of these peptides in animal models of infection and refine dosing parameters. Researchers are also engineering derivative peptides targeting other hard-to-treat pathogens and immune players.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)