Revolutionary Drug-Coated Balloon Shows Long-Term Promise for In-Stent Restenosis
2025-03-10
Author: Ming
Groundbreaking Update from Washington, D.C.
In a groundbreaking update from Washington, D.C., the breakthrough Agent drug-coated balloon (DCB) by Boston Scientific continues to demonstrate its effectiveness in treating in-stent restenosis (ISR) over a two-year period. According to recent findings, this innovative device not only enhances patient safety but also reduces the need for additional interventions compared to conventional balloon angioplasty.
Significant Findings
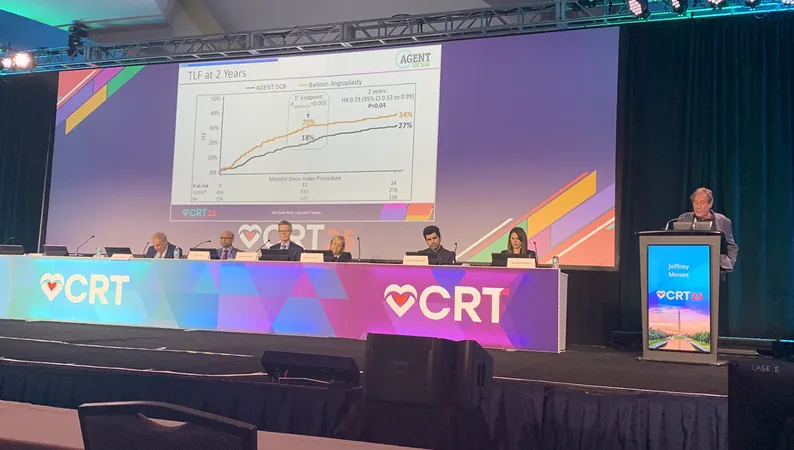
The latest data reveals that the Agent DCB yielded a target lesion failure rate of 27%, which is notably lower than the 34% observed with uncoated balloons (P = 0.04). This significant difference underscores the potential of the Agent DCB in managing ISR, a common complication following stent placement, where artery blockage reoccurs.
Expert Opinions
Dr. Jeffrey W. Moses from NewYork-Presbyterian/Columbia University emphasized the study's optimistic outcomes, highlighting a remarkable 37% relative risk reduction in target lesion failure after two years. Notably, there were no reported cases of thrombosis among patients treated with this DCB, showcasing its safety profile.
Approval and Initial Results
Approved last year, the Agent DCB is the first of its kind in the United States, utilizing a low dose of paclitaxel for effective treatment based on initial results from the AGENT IDE trial. Dr. David Cohen, a cardiovascular expert, acknowledged the encouraging two-year results but hinted that further durability and real-world effectiveness still require scrutiny.
Caution from Experts
During the Cardiovascular Research Technologies (CRT) conference, Dr. Gregg Stone cautioned that while comparing Agent DCB with standard balloon angioplasty, it is crucial to view drug-eluting stents (DES) as the gold standard for ISR treatment. He emphasized the need for ongoing studies to establish the place of DCBs in this competitive landscape.
Trial Demographics
The AGENT IDE trial enrolled 600 patients with an average age of 68, of which a significant population had multiple comorbidities, including a high prevalence of diabetes and multivessel coronary artery disease (CAD). The trial carefully selected candidates with ISR from past treatments, ensuring robust recruitment criteria to validate the DCB's efficacy.
Economic Challenges
Despite the promising findings, the high cost of the Agent DCB—approximately $5,000 in the U.S.—poses economic challenges in its widespread adoption compared to Europe, where the cost is significantly reduced. This financial burden is a critical factor for U.S. operators and patients seeking effective ISR management.
Treatment Adherence
Over the two-year period, many patients receiving the DCB maintained antiplatelet therapy, with 86% remaining on aspirin and 84% on dual antiplatelet therapy. This suggests adherence to treatment protocols, which are vital for long-term success.
Individualized Treatment Strategies
Further discussions highlighted the need for clarity in treatment strategies. Dr. James Hermiller noted that the DCB may serve as a valuable option when a patient has already received multiple stents. However, he indicated that specific scenarios vary, requiring individualized decisions.
Conclusion
The evolving landscape of ISR treatment continues to pressure clinicians and manufacturers alike to provide economical yet effective solutions. As the medical community awaits additional data and long-term follow-up, the Agent DCB holds considerable promise as a transformative tool in the fight against ISR, potentially reshaping standard practices in coronary intervention.
As we move forward, one thing is certain: the quest for optimal heart health has never been more critical, and technologies like the Agent DCB could redefine patient care in cardiology. Stay tuned as we bring you more updates on this pivotal advancement!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)