Revolutionary Discovery Could Halt Alzheimer’s with STING Molecule Blockage
2025-06-03
Author: Li
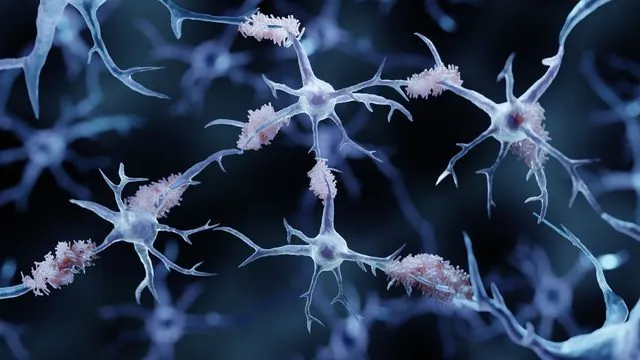
A groundbreaking revelation in Alzheimer's research suggests that halting the activity of a particular immune molecule, known as STING, could be the breakthrough needed to combat the devastating cognitive decline associated with Alzheimer’s disease and other neurodegenerative conditions.
Researchers at the School of Medicine have explored the theory that Alzheimer’s may stem from the immune system’s misguided attempts to repair DNA damage in the brain. Their studies uncovered a striking correlation: the STING molecule fuels the development of the harmful plaques and protein tangles linked to Alzheimer’s. Remarkably, when STING was blocked in laboratory mice, it resulted in a significant shield against mental decline.
STING's Role in Neurodegenerative Diseases
The findings illuminate the crucial role of STING not only in Alzheimer’s but potentially in other neurological afflictions, including Parkinson’s disease and amyotrophic lateral sclerosis (ALS). As the research progresses, treatments targeting STING could benefit a wider range of patients suffering from debilitating conditions.
John Lukens, PhD, the director of UVA’s Harrison Family Translational Research Center, states, “Our findings reveal that the DNA damage that accumulates with aging triggers STING-mediated inflammation and neuronal harm in Alzheimer’s disease.” This discovery sheds light on the heightened Alzheimer’s risk that comes with aging and highlights a new target for neurodegenerative disease therapies.
The Alarming Rise of Alzheimer’s
The statistics surrounding Alzheimer’s disease are staggering, with over 7 million Americans currently afflicted. Experts project that this number could soar past 13 million by the year 2050, prompting researchers to accelerate their efforts in understanding and treating this growing crisis.
While the precise origins of Alzheimer’s remain elusive, the emerging consensus points to the immune system playing a significant role. STING, an integral part of the immune response, originally functions to clear viruses and damaged cells. However, when it becomes overly active, it can incite harmful inflammation leading to tissue damage.
Promising Research Outcomes
In their investigations, Lukens’ team found that inhibiting STING reduced inflammation around amyloid plaques, safeguarded neighboring neurons, and improved memory in model mice for Alzheimer’s. Jessica Thanos from the University’s Department of Neuroscience noted, “Our findings suggest that STING drives harmful immune responses in the brain that contribute to cognitive decline.
Hope on the Horizon
The UVA Health researchers are optimistic about the potential of targeting STING in new therapeutic avenues. This approach appears not only to slow the buildup of amyloid plaques but also to hinder the formation of tau tangles—two primary hallmarks of Alzheimer’s.
As they delve deeper, Thanos highlighted the necessity of unearthing the intricate roles immune activation plays in both normal and aging brains. “Understanding which signals sustain that activation could enable us to intervene more effectively in diseases,” she added.
Moving forward, foundational questions remain regarding STING’s multifaceted roles, including its function in the body's response to cancer, to prevent any unforeseen side effects of new treatments.
Lukens and his dedicated team are driven by the urgent need for treatments that could stave off neuronal damage in aging brains. He emphasized, “Our aim is to edge closer to discovering safer and more effective strategies to protect against Alzheimer’s, ultimately transforming these insights into disease-modifying therapies.”

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)