Revolutionary Breakthrough: New Method to Correct Misplaced Proteins Could Transform Disease Treatment
2024-09-21
Author: Li
Exciting Development in Cellular Biology and Medicine
In an exciting development for cellular biology and medicine, researchers from Stanford University have unveiled a novel strategy for correcting the placement of proteins within cells—an innovation that holds significant promise for treating diseases such as cancer and amyotrophic lateral sclerosis (ALS).
The Role of Proteins in Cellular Functions
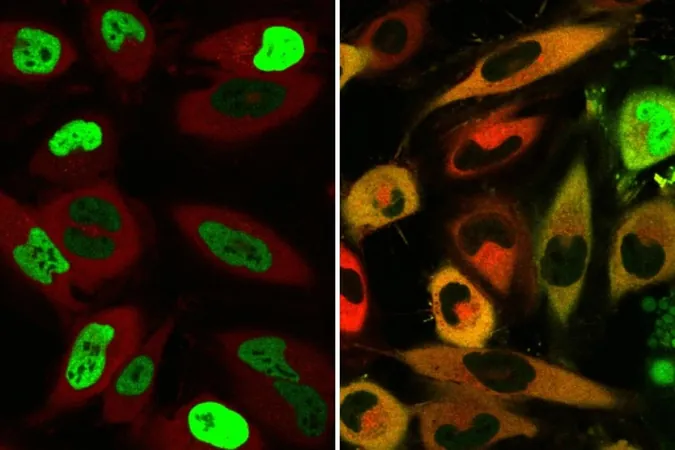
Led by the visionary scientist Steven Banik, the team has engineered a groundbreaking molecule known as TRAM (Targeted Relocation of Abnormal Molecules) that acts like a guiding system, ensuring that proteins find their way to the correct locations in the cell. This breakthrough could revolutionize the treatment landscape for various diseases linked to protein misplacement.
Understanding Protein Misplacement
Proteins play critical roles in cellular functions, including building essential molecules and transmitting signals. When proteins mislocate—akin to a package arriving at the wrong address—cellular functions can be disrupted, leading to disease progression. For example, in ALS, a protein called FUS misplaces itself and aggregates, resulting in cell death.
Mechanism of TRAM
The TRAM molecules function as helpers by binding specifically to misplaced proteins and a shuttle protein that facilitates transport. This innovative mechanism effectively guides the proteins back to their intended destinations. The research team worked with two particular shuttle systems: one for moving proteins into the nucleus—the control center of the cell—and another for expelling them.
Contributions from Graduate Student Christine Ng
Graduate student Christine Ng played a pivotal role by creating TRAMs that link the shuttle protein with the misplaced protein. However, measuring protein levels within the nucleus remained a challenge, prompting Ng to develop a new method to accurately track and quantify these cell proteins.
Successes in ALS Treatment
Ng’s experiments demonstrated that TRAMs were effective in facilitating protein relocation, leading to insights regarding optimal strength and efficiency for these transport systems. One of her notable successes involved treating ALS by directing the FUS protein back into the nucleus. This intervention not only diminished toxic aggregates but also promoted cell survival.
Expansion of Research
Further expansion of this research led to designing additional TRAMs that mimic protective mutations, promoting enhanced resilience in nerve cells by relocating specific proteins to critical areas.
Challenges Ahead
Despite the promise of this technique, the team faces a formidable challenge: accurately targeting proteins requires an understanding of the various molecules that interact with these proteins. To tackle this, they employed genetic tools to attach 'sticky tags' to the proteins, which could help identify binding sites for TRAMs.
Future Implications of TRAMs
The implications of this research extend beyond ALS and cancer. The Stanford team envisions that TRAMs might facilitate the relocation of healthy proteins to newly unexplored areas within cells, potentially unveiling entirely new biological functions. Banik expressed enthusiasm over the future potential of this technology, suggesting it might lead to discovering novel cellular processes and therapeutic approaches.
Conclusion
As researchers continue to build on these findings, the potential for TRAMs to transform how we approach protein misplacement in diseases could open new frontiers in medicine and biotechnology. The scientific community eagerly awaits the next steps as this promising research unfolds.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)