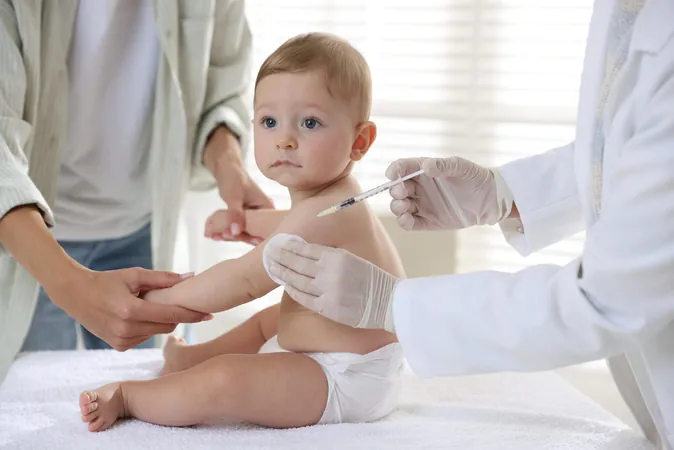
Revolutionary 21-Strain Pneumococcal Vaccine Trial Kicks Off to Shield Millions of Babies
2025-08-20
Author: Ming
Groundbreaking Vaccine Trial Begins
In a world-first initiative, the Murdoch Children’s Research Institute has embarked on a groundbreaking international trial for a 21-valent pneumococcal vaccine aimed at protecting infants from pneumonia, meningitis, and sepsis. This innovative vaccine promises to provide a broader shield against serious infections that severely threaten the health of babies worldwide.
The Need for Enhanced Protection
Currently, infants receive the 13-valent pneumococcal conjugate vaccine, PCV13, which defends against just 13 specific strains of pneumococcal bacteria linked to life-threatening diseases. Vaccination is essential for preventing these infections, which can lead to severe complications in young children, making the pursuit of more comprehensive solutions critical.
Why 21 Strains Are Better Than 13
The new vaccine under trial takes a leap forward by targeting 21 strains of pneumococcal bacteria, significantly increasing the spectrum of protection. Administered in four doses—at two, four, and six months, followed by a booster at 12-15 months—this vaccine aims to integrate seamlessly with the existing National Immunisation Program, enhancing overall immunity in the pediatric population.
Who Can Participate in This Pioneering Trial?
To ensure the safety of all participants, the study will include healthy infants aged between two months at study entry, with specific health criteria such as a full-term birth and a weight over 2.5 kg. The focus is on creating a controlled environment where the vaccine's safety and efficacy can be thoroughly evaluated.
Expert Insights on the Trial’s Importance
Professor Margie Danchin from MCRI emphasizes the importance of the trial: “Pneumococcal infections come from bacteria common in the nasal passages and can escalate into severe pneumonia or blood infections. With over 90 known strains, existing vaccines aren't comprehensive. This new trial aims to change that, potentially saving countless lives.”
A Parent's Commitment to Better Vaccines
Lisa, the mother of three-month-old Lucy, is thrilled to be part of this trial, recognizing vaccination's vital role in safeguarding her child's health. "As a nurse, I understand the importance of keeping my baby safe, especially during the winter. Trials like this are essential for discovering more effective vaccines that can protect our children in the long run,” she stated, underlining the collaborative effort between research and parental advocacy.
A Game-Changer in Infant Health
The launch of this 21-valent pneumococcal vaccine trial marks a significant milestone in pediatric healthcare. By broadening the protective umbrella against pneumococcal diseases, researchers hope to create a future where infants worldwide can thrive free from the fear of these dangerous infections.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)