New Study Reveals How Timing of Stopping Tenofovir Affects Postpartum Liver Health and Breastfeeding in Hepatitis B Moms
2025-08-31
Author: Rajesh
Hepatitis B virus (HBV) infection is a pressing global health challenge, impacting around 240 million individuals worldwide. One of the primary routes of transmission is from mothers to their newborns, with about 5.5% of women of childbearing age in China testing positive for the virus. While immunoprophylaxis can successfully protect over 90% of newborns from HBV, a small percentage still develops chronic hepatitis B, making antiviral treatments crucial.
The Role of Tenofovir in Preventing Transmission
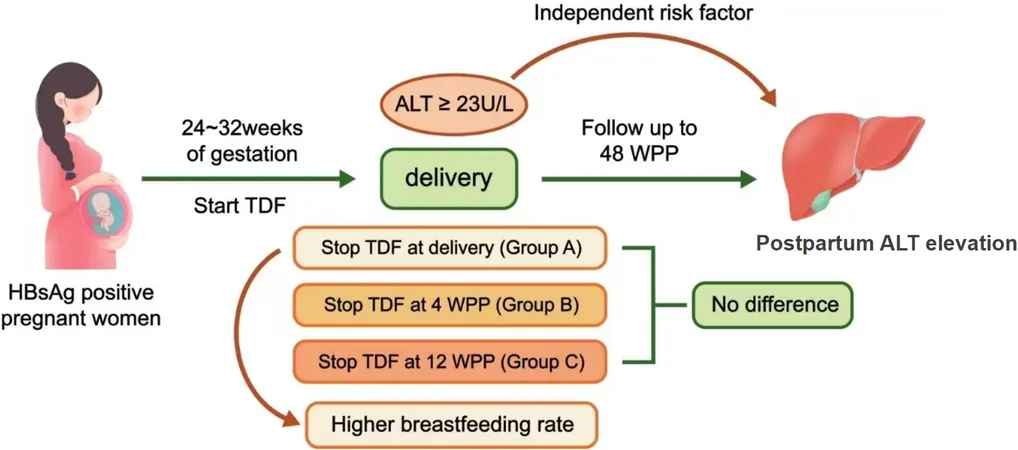
Tenofovir disoproxil fumarate (TDF) is recognized as the first-line antiviral treatment for preventing mother-to-child transmission during pregnancy due to its efficiency and safety for both mothers and infants. Guidelines across various health organizations suggest starting TDF during the second or third trimester and propose different timelines for discontinuing the medication postpartum.
Understanding the Impact of Stopping Antiviral Treatment
A recent study aimed to shed light on the effects of stopping TDF at different postpartum stages. Researchers explored how the timing of TDF withdrawal influences the risk of elevated alanine aminotransferase (ALT) levels in mothers, which could indicate liver inflammation, and examined breastfeeding outcomes.
Key Findings and Insights
Conducted in Hangzhou, China, the prospective study involved pregnant women with chronic hepatitis B, monitoring their ALT levels and breastfeeding rates after stopping TDF. Three groups were created based on withdrawal timing: at delivery, 4 weeks postpartum, or 12 weeks postpartum. Notably, while stopping TDF didn’t significantly affect overall ALT levels, an early withdrawal was linked to higher breastfeeding rates—79.7% of mothers who stopped at delivery opted to breastfeed.
ALT Levels and Liver Health
During the first 48 weeks postpartum, over 56% of participating mothers experienced elevated ALT levels, with the peak incidence occurring around 6 weeks postpartum. Interestingly, the group that continued TDF for 12 weeks displayed significantly lower HBV DNA levels and a reduced incidence of elevated postpartum ALT, suggesting that extended treatment could enhance liver health.
Breastfeeding Trends Among Mothers
Despite the recommendation to continue breastfeeding while on TDF, perceptions around medication safety led many mothers to shy away from breastfeeding post-treatment. The disparity in breastfeeding rates between the groups underscores the necessity for ongoing patient education and alleviating misconceptions surrounding antiviral medications and breastfeeding.
Future Directions: A Call for Expanded Research
While this study provides valuable insights into the management of HBV during pregnancy, it also brings to light the complexities of individualized care for mothers with chronic hepatitis B. The variations in ALT levels and breastfeeding practices indicate a need for future large-scale studies to validate these findings and refine treatment strategies tailored to maternal health.
Ultimately, this research emphasizes the importance of not only managing hepatitis B effectively but also ensuring that breastfeeding needs are met, striking a balance between maternal health and infant welfare.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)