
New Study Reveals Anti-CGRP Monoclonal Antibodies Pose No Greater Cardiovascular Risk for Migraine Sufferers
2025-01-15
Author: Mei
Study Overview
Recent research has debunked the myth that anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) could increase cardiovascular (CVD) risks in adults suffering from migraines. This study addresses previous skepticism regarding their potential cardiovascular implications.
Research Leadership and Insights
Led by Dr. Seonkyeong Yang from the University of Florida, the research provides critical insights into the safety of anti-CGRP treatments in real-world scenarios. "Our comprehensive analyses yield consistent findings, broadening our understanding of cardiovascular safety among populations frequently overlooked in randomized controlled trials (RCTs), particularly older adults and individuals with disabilities," the study noted.
Concerns Addressed
Despite their effectiveness as preventive treatments for migraines, concerns had been raised that these mAbs might exacerbate existing hypertension or lead to new hypertensive events, and even escalate transient ischemia into serious outcomes like myocardial infarctions or strokes.
Study Methodology
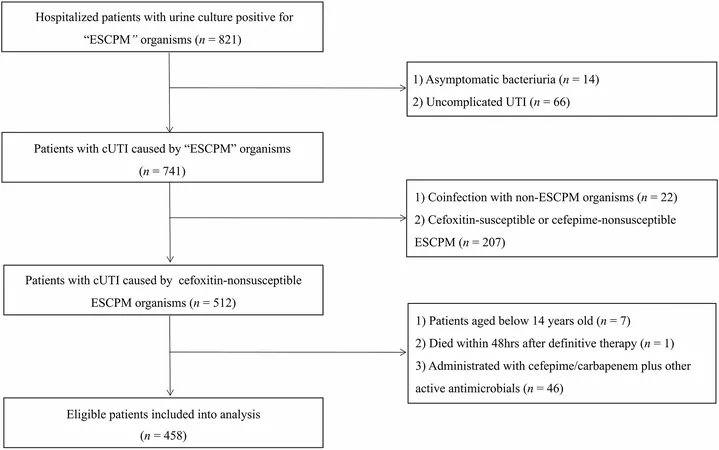
In a rigorous study design, researchers conducted a retrospective cohort analysis focusing on 266,848 patients with migraines who began treatment with either anti-CGRP mAbs or onabotulinumtoxinA from May 2018 to December 2020. The objective was to evaluate and compare the incidence of cardiovascular complications in these groups while minimizing biases related to the introduction of new medications and the impact of the COVID-19 pandemic.
Participant Criteria
Participants included Medicare beneficiaries over 18 years old who had a history of migraines and started treatment during the study period. Those with previous cardiovascular events or certain medical conditions were excluded to avoid skewed results.
Endpoints and Outcomes
The primary endpoint was the incidence of myocardial infarction or stroke, while secondary outcomes included hypertensive crises and other vascular conditions.
Study Demographics
Among patients treated with anti-CGRP mAbs (5,153 participants), the average age was 57.8 years, with a significant female majority (83.6%). Erenumab emerged as the leading CGRP inhibitor, making up 60.4% of the medications, followed by galcanezumab and fremanezumab. In the alternative treatment group using onabotulinumtoxinA (4,000 participants), the average age was slightly higher at 61.9, with a comparable gender distribution.
Interesting Findings
The study unveiled interesting demographics, revealing that those on anti-CGRP mAbs were more likely to report disabilities and obesity, but exhibited a lower prevalence of pain conditions like fibromyalgia compared to the onabotulinumtoxinA group.
Key Results
Crucially, the results indicated comparable rates of composite CVD outcomes: 5.83 incidents per 1,000 person-years for anti-CGRP mAb users versus 6.81 for onabotulinumtoxinA users. Indeed, the risk of composite CVD events (adjusted hazard ratio of 0.88) and serious complications like hypertensive crises (aHR of 0.46) remained unaffected by anti-CGRP treatments.
Significant Findings on Cardiovascular Events
Moreover, participants who began anti-CGRP treatments did not demonstrate a significant increase in individual cardiovascular events, including myocardial infarction or stroke.
Conclusion and Future Research
These findings reinforce the idea that real-world observational studies are vital for gaining comprehensive insights into the safety profiles of medications like anti-CGRP mAbs. The researchers underscored the necessity for further exploration into the long-term effects of such treatments, particularly in larger and more diverse populations.
Stay Informed
This groundbreaking study paves the way for migraine sufferers to feel safe in their treatment options, but future research will be crucial to confirm these findings and establish comprehensive long-term safety data.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)