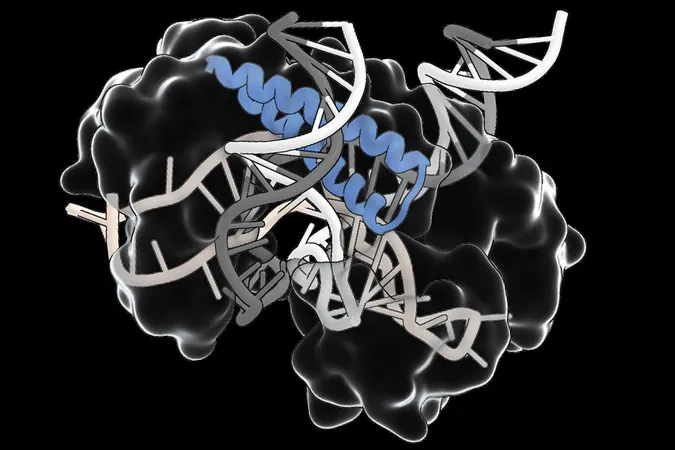
Is this New RNA System Set to Dethrone CRISPR in Gene Editing?
2025-04-09
Author: Siti
Introduction
In a groundbreaking revelation, researchers at MIT and the Broad Institute have unearthed an innovative class of RNA-guided DNA targeting systems, dubbed TIGR-Tas. This discovery stands apart from the widely known CRISPR-Cas systems, boasting unique structural features, a compact size, and a different approach to targeting that negates the need for specific DNA ‘anchors’ known as PAM sites. These advancements could potentially streamline various aspects of genome engineering, offering simplified delivery methods for cell and gene therapies that can broaden the spectrum of genetic diseases treatable through gene editing.
Versatility and Implications
As noted in a recent article in Science, lead investigator Feng Zhang expressed confidence in the versatility of the TIGR-Tas system, stating, "This is a very versatile RNA-guided system with a lot of diverse functionalities." The implications of this system are staggering; it could redefine therapeutic strategies for conditions previously deemed uneditable.
Discovery Process
The journey to unveiling TIGR-Tas commenced with a strategic computational search highlighting a crucial component of the Cas9 protein – its guide RNA-binding domain. Researchers scoured extensive databases for prokaryotic and viral proteins, ultimately employing an AI-driven protein language model that clustered candidates based on structural features and evolutionary lineage. This meticulous process led to the identification of a distinct group of proteins linked to regularly spaced, repetitive DNA sequences, reminiscent of CRISPR arrays.
Collaboration and Multidisciplinary Effort
This significant discovery stemmed from a collaborative effort at the McGovern Institute for Brain Research at MIT and the Broad Institute, with key contributions from experts like Guilhem Faure, Makoto Saito, Max Wilkinson, Rhiannon Macrae, and Eugene Koonin. Their multidisciplinary approach illustrates the potential of combining diverse scientific backgrounds to drive innovation.
Diversity of Tas Proteins
The exploration yielded over 20,000 different Tas proteins within this novel family, primarily found in bacteriophages and parasitic bacteria. This remarkable variety suggests that these proteins may play diverse roles in their natural habitats, which researchers are now keen to investigate further.
Potential for Gene Therapy and Targeting
The compact size of TIGR-Tas is particularly promising for cell and gene therapy applications, alleviating a significant barrier in packaging genetic material into viral vectors, such as AAVs, which are commonly used in therapies. Additionally, the lack of PAM site requirements could open up new avenues for targeting previously inaccessible areas of the human genome, making it easier to edit disease-causing mutations.
Modularity and Bioengineering Opportunities
Beyond therapeutic use, the modularity of TIGR-Tas presents an exciting opportunity for bioengineering. Researchers foresee the possibility of integrating different functional domains into the Tas RNA-binding scaffold, paving the way for the creation of novel editing tools like base or epigenome editors. Furthermore, as a standard research tool, it could transform fundamental biology studies and deepen our understanding of natural roles in microbial ecosystems and the evolution of RNA-guided mechanisms.
Challenges Ahead
However, despite its potential, TIGR-Tas is still in its infancy, having only been discovered recently (with its findings published in February 2025). Unlike the mature CRISPR toolkit, which has undergone extensive refinement, TIGR-Tas needs thorough validation regarding its genome-wide specificity. While preliminary assessments suggest its dual-guide mechanism may enhance precision, concerns linger regarding the possibility of unintended off-target effects due to its lack of PAM site dependence. It is imperative for the broader research community to conduct comprehensive experiments to clarify these ambiguities.
Optimization for Therapeutic Use
Moreover, optimizing the efficiency of TIGR-Tas for therapeutic use will be crucial. This includes tackling challenges of in vivo delivery to target tissues and addressing potential immune responses to the Tas proteins derived from bacteria and phages. The road ahead requires collaborative efforts to validate this promising technology while the original research team continues to focus on its structural biology, performance-enhancing protein engineering, and understanding the system's natural functions.
Conclusion
In summary, TIGR-Tas stands at a pivotal point with the potential to revolutionize gene editing. As further research unfolds, the scientific community eagerly anticipates whether this novel system can truly challenge the reign of CRISPR technology. Could we be witnessing the dawn of a new era in genetic engineering?




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)