Groundbreaking Study Reveals How Genetic Variants Fuel Alzheimer's Inflammation in Women!
2024-09-30
Recent research conducted by investigators at Weill Cornell Medicine has uncovered alarming insights into how two genetic variants linked to Alzheimer's disease (AD) activate a detrimental inflammatory response in the brain's immune cells, particularly affecting women. This preclinical study, published on September 30 in the journal Neuron, highlights the urgent need to address sex differences in Alzheimer's research to lead to more precise and effective therapies.
Alzheimer's disease currently afflicts millions across the globe, with women being disproportionately impacted—almost twice as many females develop this devastating disease compared to males. This significant disparity has led researchers to delve deeper into the causes behind women's increased vulnerability, a key focus of the current study.
The researchers targeted the APOE4 gene variant, known for its heightened risk of AD, which seems to affect women more severely than men. They also explored the implications of another variant of the TREM2 gene, which similarly elevates Alzheimer's risk. Despite previous knowledge of these factors, their combined effects had not been thoroughly studied until now.
Dr. Li Gan, the lead researcher and director of the Helen and Robert Appel Alzheimer's Disease Research Institute, noted, “These two variants are amongst the strongest known risk factors for Alzheimer's, yet their mechanisms remain poorly understood. Our goal was to examine what happens at a cellular level when both of these risk factors are present in females.”
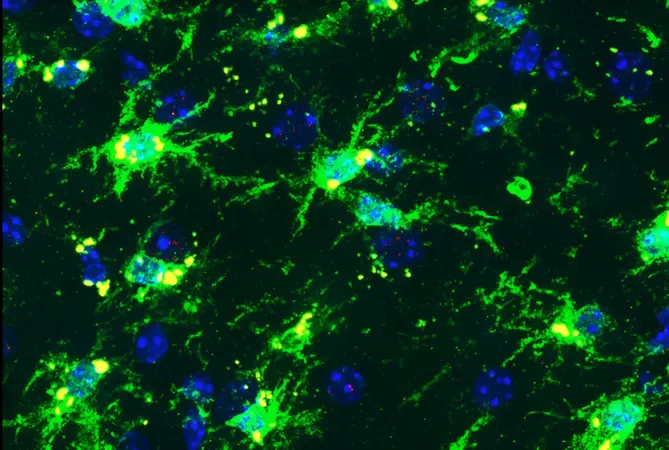
To investigate this, Dr. Gan's team, with lead author Dr. Gillian Carling, created mouse models of AD that carried human versions of the APOE4 and a rare TREM2 variant known as R47H, which significantly increases Alzheimer's risk. With an added mutation linked to tau protein clumping—a key feature in Alzheimer’s affecting cognitive function—the research team provided critical insights by studying the mice at a stage of development equivalent to middle age in humans.
The study revealed striking findings: female mice with both genetic variants suffered considerable damage to brain regions essential for memory and thought. In contrast, their male counterparts exhibited no such deterioration. This discrepancy was attributed to dysfunction in microglia, the brain's immune cells. Under normal conditions, microglia would clean up neurotoxic debris, but in this scenario, they aged prematurely and ceased their protective functions, instead releasing harmful inflammatory chemicals.
Importantly, the research emphasized the specific vulnerability of female microglia, aligning with existing data that show APOE4 poses a stronger risk for women. Dr. Carling commented on the findings, stating, "Our study revealed that the combination of these Alzheimer's risk factors activated the cGAS-STING pathway to a dangerous extent in female mice, which exacerbates inflammation."
In a hopeful twist, the researchers noted that inhibiting the cGAS-STING pathway mitigated harmful inflammatory responses and improved microglial function, highlighting potential new treatment avenues.
This study underscores the importance of understanding sex differences in Alzheimer's progression, advocating for tailored approaches in research and therapies. With hope for future advancements, the researchers aim to further explore immune mechanisms like cGAS-STING as potential targets for intervention to combat Alzheimer’s, especially for individuals carrying high-risk genetic variants.
As these pivotal discoveries emerge, they represent a significant step towards not only understanding but also battling Alzheimer's disease with strategies that account for the unique challenges faced by women. Stay tuned, as breakthroughs in this critical area of research could revolutionize treatment options within the coming years!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)