Groundbreaking scRNA-seq Atlas Unveils Myeloma's Immune Microenvironment: A Game Changer for Future Therapies!
2024-11-13
Author: Daniel
Groundbreaking scRNA-seq Atlas Unveils Myeloma's Immune Microenvironment: A Game Changer for Future Therapies!
In a remarkable leap forward, scientists from VIB and VUB have unveiled a detailed single-cell RNA-sequencing (scRNA-seq) atlas of the immune microenvironment in multiple myeloma (MM), a challenging and often incurable blood cancer. This innovative resource provides crucial insights into the disease's progression and offers a pivotal tool for researchers striving to develop effective immunotherapies.
Multiple myeloma is notorious for its harsh impact on patients, with serious ramifications including weakened immunity and bone damage. The relentless nature of the disease is highlighted by the high rates of relapse after initial treatments, intensifying the imperative for novel therapeutic strategies. "The high relapse rates signal an urgent need for innovative immunotherapies that can rejuvenate the immune response," emphasizes Dr. Damya Laoui from the VIB-VUB Center for Inflammation Research.
The new interactive tool created by the researchers is set to transform the landscape of MM treatment by facilitating immune-based patient stratification. “Our comprehensive immune atlas tracks the evolution of myeloma from early stages through progressive phases,” Dr. Laoui explains. This cutting-edge resource is freely accessible and stands to enhance the pace of research into durable immunotherapeutic strategies.
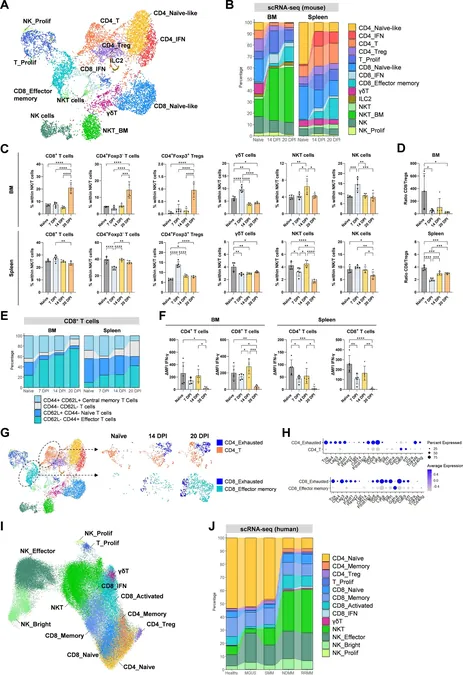
Significantly, the unique architecture of the multiple myeloma tumor microenvironment (TME) poses a critical barrier to treatment success. This research addresses that challenge, offering an in-depth examination of the immune dynamics at play. Through collaboration among various research teams, Emma Verheye and her colleagues mapped these dynamic changes in an immunocompetent MM mouse model, revealing potential resistance mechanisms that hinder effective therapy outcomes.
Verheye highlights, “Our findings correlate the immune changes observed in our murine models with those in human patients, confirming the accuracy of this mouse model in representing the human condition across disease stages.” This correlation is vital for fine-tuning immunotherapies to match the evolving landscape of the disease.
The researcher’s findings illustrated a pronounced increase in T cells exhibiting exhausted phenotypes within the MM-TME. Interestingly, while neutrophils appeared harmless in the early disease stages, they gained pro-tumorigenic traits as the disease progressed. Furthermore, conventional dendritic cells (cDCs) showed reduced activity, indicating that boosting immune responses could be a promising therapeutic avenue.
Dr. Kim De Veirman notes, “Our tool has identified cDCs as a population that can be targeted in MM. We have carried out the first pre-clinical evaluation of DC-activating αCD40 therapy using both murine and human samples." The administration of αCD40 not only activated cDCs but also instigated significant T-cell activation, culminating in a remarkable short-term anti-tumor response.
These groundbreaking results establish the newly developed research tool as a vital asset for the MM research community, opening pathways for further therapeutic innovations. By shedding light on the complexities of the multiple myeloma immune microenvironment, this study paves the way for more personalized and effective treatment options for patients battling this formidable illness. As the science progresses, the hope for durable and effective immunotherapies inches closer to reality!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)