Groundbreaking Discovery Reveals Simple Rules Behind Protein Stability Mutations – What It Means for Disease Treatment and Industry!
2024-09-25
Introduction
In a revealing study published in the prestigious journal *Nature*, a team of researchers from the Center for Genomic Regulation (CRG) and the Wellcome Sanger Institute has unearthed a remarkable insight: mutations affecting protein stability follow surprisingly simple mathematical rules. This groundbreaking discovery is set to revolutionize the development of innovative therapies for a multitude of diseases and aid in the engineering of proteins for industrial purposes.
Understanding Protein Mutations
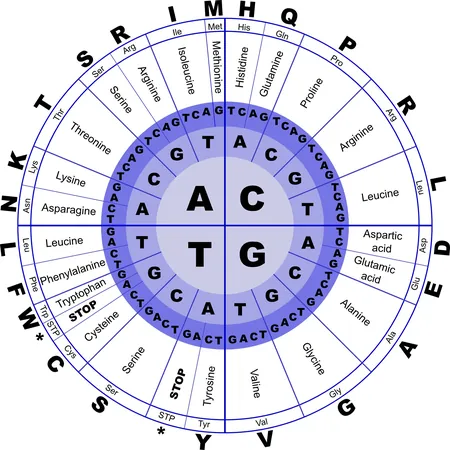
Proteins, which are vital for nearly every biological process, consist of chains made up of twenty different amino acids. Even a single mutation—where one amino acid swaps for another—can significantly alter the protein's structure, potentially leading to health issues, including cancer and neurodegenerative diseases. Understanding how these mutations influence protein shape is crucial for unraveling their roles in various diseases.
The Challenge of Protein Stability
The sheer complexity of potential mutations poses a daunting challenge for researchers. For instance, a protein that is just 34 amino acids long can have a staggering 17 billion different combinations if only one position is allowed to change. Testing each of these combinations in a lab setting is not only time-consuming but practically impossible; if each test took a mere second, researchers would need 539 years to evaluate all possible variants.
As proteins become longer, the possible mutation combinations grow exponentially, surpassing even the number of atoms in the universe for lengthy proteins typical in human biology. Despite this daunting landscape, the collaborative work led by Dr. André Faure and ICREA Research Professor Ben Lehner has established that the influence of mutations on protein stability is far more predictable than previously assumed.
New Insights from Research
Historically, scientists worried about unpredictable interactions between mutations, which could amplify or suppress each other's effects. However, this new research indicates that while some interactions do occur, they are infrequent, and most mutations operate independently. As Dr. Lehner states, 'Our discovery turns an old understanding on its head, showing that the endless possibilities of protein mutations boil down to straightforward rules.'
The team achieved this breakthrough by creating thousands of protein variants, each with unique mutation combinations. They then meticulously assessed the stability of these proteins, yielding extensive data on how individual mutations and their combinations affect protein integrity. Their findings closely aligned with simpler models suggesting that the cumulative impact of several mutations can be effectively estimated by summing up the effects of individual mutations.
Implications for Disease Treatment
This clarity has profound implications for the understanding and management of genetic diseases. With many disorders arising from multiple mutations within a single protein, the ability to predict how specific combinations of mutations influence a protein’s stability could enhance diagnostic accuracy and facilitate more personalized treatment strategies.
Impact on Drug Development
Moreover, the study paves the way for more efficient drug development. For instance, researchers could better target misfolded proteins, as seen in Alzheimer’s disease, where abnormal amyloid-beta proteins contribute to damaging plaque formation in the brain. By identifying and stabilizing particularly destabilizing mutations, new therapeutics could be designed to restore protein function.
Biotechnological Applications
The findings also offer exciting possibilities for biotechnological applications. For example, enzymes capable of breaking down environmental plastics could be improved through strategic mutation, enabling the creation of more effective and stable engineered proteins to tackle pollution.
Conclusion and Future Directions
While this study marks a significant advance, the researchers acknowledge limitations, such as the potential for complex interactions involving three or more mutations that aren't accounted for simply by adding individual effects. Additionally, while these novel rules can streamline research efforts, some degree of experimental validation will always be necessary to verify predictions, especially in drug development where rare interactions may still occur.
As this research unfolds, the implications reach far and wide, promising to unlock new doors in medicine and industry alike. Keep an eye on this developing story, as it unfolds a potential revolution in our understanding of biochemistry and its applications to real-world problems!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)