Groundbreaking Discovery Links "Guardian of the Genome" to Cancer Risk in Ulcerative Colitis
2024-10-28
Author: John Tan
Introduction
Researchers at the Max Delbrück Center and Charité – Universitätsmedizin Berlin, under the guidance of Michael Sigal, have unveiled significant new insights regarding the pivotal role of the p53 gene in ulcerative colitis (UC). Their study, published in *Science Advances*, unveils an exciting potential pathway for developing new drug treatments aimed at halting the progression of this inflammatory condition to colon cancer, a disease that currently affects roughly five million individuals globally.
Role of p53 in Ulcerative Colitis
Led by graduate student Kimberly Hartl, the team highlighted how p53, known as the "guardian of the genome," plays a crucial role in the pathogenesis of UC, which significantly increases the risk of developing colorectal cancer. Professor Sigal explained, “In patients with ulcerative colitis who are at high risk for developing cancer, we could potentially target aberrant cells and eliminate them before cancer even occurs.”
Understanding the Mechanism of Injury and Repair
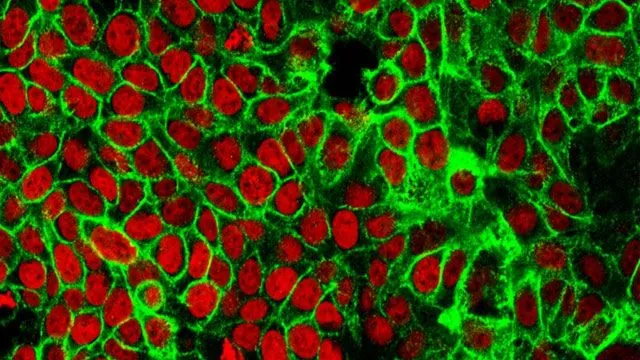
Ulcerative colitis primarily impacts the large intestine and disrupts the function of epithelial crypts—tube-like glands that are essential for maintaining intestinal health. Following injury to the colon, these crypt cells go into a “repair mode,” rapidly proliferating to address the damage. However, in patients with UC, this healing process can malfunction, causing cells to become stuck in what is termed a “regenerative cell state.” This dysfunctional state leads to a lack of mature cells and initiates a detrimental cycle of increased stem cell proliferation.
Research Findings
The study revealed that this problematic repair process is closely tied to a non-functional p53 gene, pivotal for regulating cellular repair and the cell cycle. “Without p53, cells remain in a continuously proliferative state,” Sigal noted.
Advancements in Detection Methods
Traditional diagnostic methods, such as colonoscopies, can identify precancerous lesions but often struggle with less visible abnormalities. This research lays the groundwork for potential molecular tools that could allow for earlier detection of aberrant cells, significantly before visible symptoms arise.
An Innovative Approach to Understanding Colonic Repair
To further understand the repair mechanisms, the research team created a three-dimensional organoid model of the colon using mouse stem cells. This innovative approach enabled them to observe that organoid cells missing p53 remained trapped in a regenerative state and exhibited heightened glucose metabolism through glycolysis—essential for rapid cell division. Conversely, when p53 is active, it may reduce glucose metabolism and guide cells back to a healthy, mature state.
Potential Therapeutic Targets
Significantly, the researchers identified that these p53-deficient organoids showed increased vulnerability to treatments targeting glycolysis, thereby opening doors to potentially new therapeutic options aimed at specifically targeting mutated cells. “With organoids, we can pinpoint specific compounds that affect metabolic pathways that may lead us to novel therapies,” Hartl commented.
Future Directions
The researchers now aim to translate these findings into clinical settings, focusing on developing straightforward methods to detect dysfunctional p53 cells in human colon tissues. Sigal emphasized, “Once we have a straightforward identification method for these individual cells, we can initiate clinical studies aimed at selectively eliminating them and evaluate whether this action correlates with a reduced risk of cancer development.”
Conclusion
With ongoing research aimed at understanding how to harness the power of p53 in therapies for ulcerative colitis, this landmark study paves the way for innovative strategies in the battle against colon cancer linked to this chronic condition. As scientists continue to explore the intricacies of the p53 gene and its role in cellular repair, who knows what groundbreaking developments await us in the future?

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)