Game Changer for Hydrogen Production: Inexpensive Nickel Compounds Poised to Replace Rare Iridium!
2024-10-28
Author: Wei Ling
Game Changer for Hydrogen Production: Inexpensive Nickel Compounds Poised to Replace Rare Iridium!
In a groundbreaking development from a collaboration between the Technical University of Berlin, HZB, IMTEK (University of Freiburg), and Siemens Energy, researchers have unveiled a highly efficient alkaline membrane electrolyzer that rivals the performance of traditional proton-exchange membrane (PEM) electrolyzers. The highlight of this innovative electrolyzer is its use of cheap nickel compounds as an anode catalyst, effectively replacing the expensive and scarce metal, iridium.
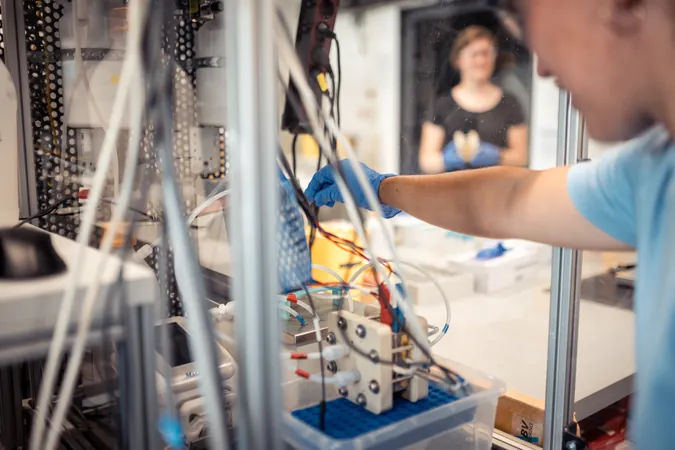
This exciting advancement was made possible through detailed operando measurements conducted at BESSY II, the Berlin synchrotron radiation source. In partnership with a theoretical team based in the U.S. and Singapore, researchers crafted a cohesive molecular description of the catalytic processes involved. Through this collaboration, prototype cells were also fabricated and rigorously tested, showcasing a new coating process that significantly enhances operational efficiency. The findings have been documented in a prestigious publication, Nature Catalysis.
As we pivot towards a more sustainable energy future, hydrogen is set to emerge as a critical player. It holds promise not only as an energy storage medium but also as a clean fuel and a crucial raw material for the chemical industry. Hydrogen production via electrolysis of water could dramatically reduce carbon emissions when powered by renewable energy sources like solar and wind.
Currently, the green hydrogen production landscape is primarily dominated by two systems: the well-established proton-conducting membrane electrolysis (PEM) and the conventional liquid alkaline electrolysis. Alkaline membrane (AEM) electrolyzers fuse the benefits of both technologies, notably eliminating the need for rare precious metals such as iridium.
The research teams from TU Berlin and HZB have now broken new ground by presenting the first AEM electrolyzer that delivers hydrogen production efficiencies comparable to those of PEM systems. By utilizing nickel double hydroxide compounds in conjunction with iron, cobalt, or manganese, they have engineered a method to apply these materials directly onto an alkaline ion exchange membrane.
The team’s work at BESSY II enabled them to gain critical insights into the molecular mechanisms of electrolysis. Prof. Peter Strasser from TU Berlin elaborated, "Our research illuminated the catalytic-chemical processes occurring at the catalyst-coated membrane, particularly the transformation from a catalytically inactive alpha phase to a highly active gamma phase. Additionally, we identified the pivotal roles played by various O ligands and Ni4+ centers in catalysis."
What sets this gamma phase apart is its competitive edge against current leading iridium catalysts. "While there are intriguing similarities in the catalytic mechanisms with iridium, we also discovered some unexpected molecular differences that could open new avenues in catalyst design and optimization," Strasser emphasized.
This revolutionary approach could transform the hydrogen production landscape by making it more affordable and accessible, allowing for rapid scaling of green hydrogen technology. With the race towards a sustainable hydrogen economy heating up, this innovation represents a crucial step toward making hydrogen a viable and economically feasible alternative for future energy systems. Could this be the breakthrough we've been waiting for? Only time will tell!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)