
Could "Smart Antibiotics" Transform Our Fight Against Infections and Protect Our Gut Health?
2024-09-24
Introduction
This week, world leaders at the UN General Assembly will gather to confront the looming crisis of antimicrobial resistance, a growing threat that could derail global health initiatives and undermine food security. This urgent meeting follows a shocking report from the Global Leaders Group on antimicrobial resistance, released in April, which tied drug-resistant infections to approximately 1.27 million deaths annually. The findings warned that without escalated efforts to combat antibiotic resistance, healthcare expenses could soar to a mind-boggling $412 billion by 2035, alongside an additional loss of $443 billion due to decreased workforce productivity.
The Alarm on Antibiotic Resistance
As antibiotic resistance emerges as a critical public health issue, the World Health Organization (WHO) has repeatedly sounded the alarm. Unfortunately, the development pipeline for new antibiotics is alarmingly sparse. Between 2017 and 2021, only 12 new antibiotics received approval, and shockingly, just one was effective against all pathogens categorized as critical by the WHO.
Impact on Gut Health
A significant factor contributing to the stagnation in antibiotic innovation is the overprescribing of these drugs, which decimates not only harmful bacteria but also the beneficial microbes residing in our gut, known as commensal bacteria. Research illustrates that the antibiotic fluoroquinolone, for instance, drastically lowers the diversity and balance of gut microbiota, leading to potential long-term health issues.
Professor Hergenrother's Contribution
Professor Paul Hergenrother from the University of Illinois Urbana-Champaign, who is at the forefront of novel antibiotic research, emphasizes the growing body of evidence linking the disruption of gut microbiomes to various health problems, including colorectal cancer, irritable bowel syndrome, and even mental health issues such as depression and diminished motivation to exercise.
The Concept of Smart Antibiotics
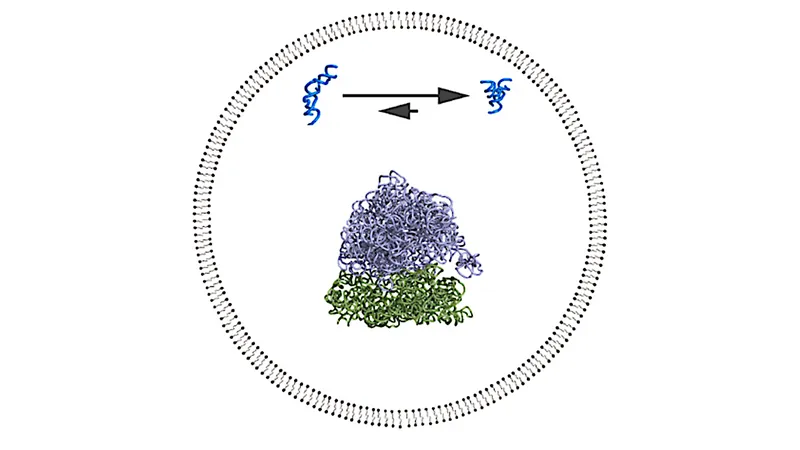
In light of these challenges, innovative solutions are on the horizon. Hergenrother is pioneering the concept of "smart antibiotics"—targeted antibiotics designed to eradicate harmful pathogens while leaving the beneficial gut bacteria unharmed. This innovative approach hinges on the discovery that certain proteins essential for the survival of pathogenic bacteria differ significantly from those in commensal species. By identifying these proteins through genetic sequencing, researchers can cultivate targeted therapies that specifically disrupt the life cycles of harmful microbes.
Groundbreaking Developments
Earlier this year, Hergenrother's team unveiled their groundbreaking antibiotic, lolamicin, which selectively annihilates virulent Gram-negative bacteria, the most notorious and drug-resistant pathogens such as E. coli. In preclinical trials involving mice, lolamicin demonstrated remarkable efficacy in treating infections without harming the gut microbiome—a significant breakthrough for the field.
Challenges in the Pharmaceutical Industry
However, despite potential advances in smart antibiotics, the pharmaceutical industry's commercial landscape remains bleak. Henry Skinner, CEO of the AMR Action Fund, highlights that low market prices and strict usage guidelines deter drug developers from producing new antibiotics. The high cost, estimated at around $1 billion per new antibiotic, culminates in minimal return on investment, causing many pharmaceutical giants to pivot away from antibiotic research towards more lucrative fields such as oncology or neurodegenerative diseases.
Funding Proposals
In February, a report from the Antimicrobial Industry Alliance further revealed that antibiotic development is increasingly relegated to small- and mid-sized companies, which often struggle financially due to production and distribution costs that far exceed their profits from low-volume sales.
Concluding Remarks
To fortify the antibiotic pipeline, Skinner advocates for the adoption of new funding mechanisms by wealthier nations, akin to the UK's antimicrobial subscription model. By ensuring returns for antibiotic development, such initiatives could encourage private sector investment and greater innovation in this crucial area.
Looking to the future, Hergenrother is keen on collaborating with commercial partners to bring lolamicin through the patent process and into clinical trials, while also modifying the compound to counteract potential bacterial resistance. He remains optimistic that the next generation of smart antibiotics will not only stand out in terms of efficacy but also mitigate adverse effects on gut health, thereby enhancing patient outcomes.
As the world grapples with an imminent healthcare crisis due to antibiotic resistance, the emergence of smart antibiotics may just be the scientific breakthrough we desperately need. Will these innovative treatments revolutionize our approach to infections and preserve our gut microbiomes? Only time will tell, but the stakes could not be higher.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)