Breakthrough Study Uncovers Key Proteins Linked to Congenital Developmental Disorders – What You Need to Know!
2024-09-27
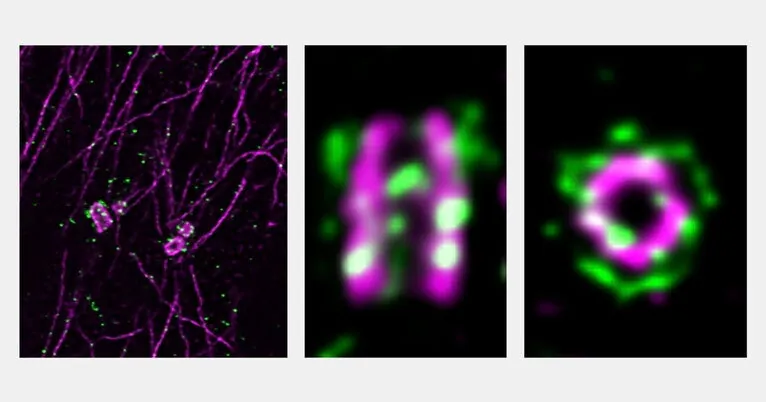
Recent research from Yale University has spotlighted the crucial roles of centrosomes—tiny cell structures that play vital roles in cell signaling and the organization of the cytoskeleton. Dysfunction in these centers has been implicated in a range of diseases, including cancer and various congenital developmental disorders. Understanding what regulates centrosome function is essential for unraveling these complex diseases.
In a groundbreaking study published in the journal Current Biology, Yale researchers have identified two proteins, PPP2R3C and MAP3K1, that are pivotal in regulating centrosome functions. This discovery not only enhances our understanding of centrosome-related disorders but also opens the door to potential new treatment pathways.
The researchers initially came across the protein PPP2R3C during a comprehensive genome-wide screening, where they systematically mutated genes to identify those that influenced centrosome function. They found that mutations in the PPP2R3C gene are linked to several developmental syndromes.
Senior author David Breslow, an assistant professor at Yale, remarked, “We were intrigued by this connection to disease. By diving into available data, we began to clarify what PPP2R3C’s role is within the cells.” Their research revealed that PPP2R3C accumulates at centrosomes in healthy cells, indicating its significance in normal centrosome function. However, the effects of knocking out this gene varied widely; some cells perished while others remained unharmed, prompting further investigation.
Breslow and his team discovered that the expression of another gene, MAP3K1, correlated with the severity of the effects seen when PPP2R3C was removed. The two proteins were found to possess opposing functions at centrosomes, akin to a car's brake (PPP2R3C) and gas pedal (MAP3K1). Absence of just the brake led to significant cell death and defects, yet removing both proteins preserved cell function.
Further reinforcing their findings, mutations in MAP3K1 also appeared to be implicated in human developmental syndromes exhibiting symptoms similar to those caused by PPP2R3C mutations. “The loss of function in PPP2R3C and gain of function in MAP3K1 lead to comparable symptoms,” Breslow explained. “This supports the idea that these proteins counterbalance one another, both contributing to an essential equilibrium.
Significantly, when the research team introduced a mutation in PPP2R3C associated with developmental syndromes into cells, they observed that the protein did not localize to the centrosomes as expected. This indicates a disruption in centrosome function and marks the first instance of such dysfunction being linked to impaired gonadal development—a key symptom related to deficiencies in both PPP2R3C and MAP3K1.
“With our findings, we may broaden the types of diseases that are recognized to stem from centrosome dysfunction,” Breslow suggested.
While centrosome dysfunction has long been linked to cancer pathologies, this research may highlight new therapeutic targets for treating cancer. Notably, neuroblastomas and B cell leukemias have shown significant dependence on the PPP2R3C protein.
“Targeting PPP2R3C with drugs that inhibit its function, or enhance the action of MAP3K1, represents a promising new avenue for therapeutic strategies,


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)