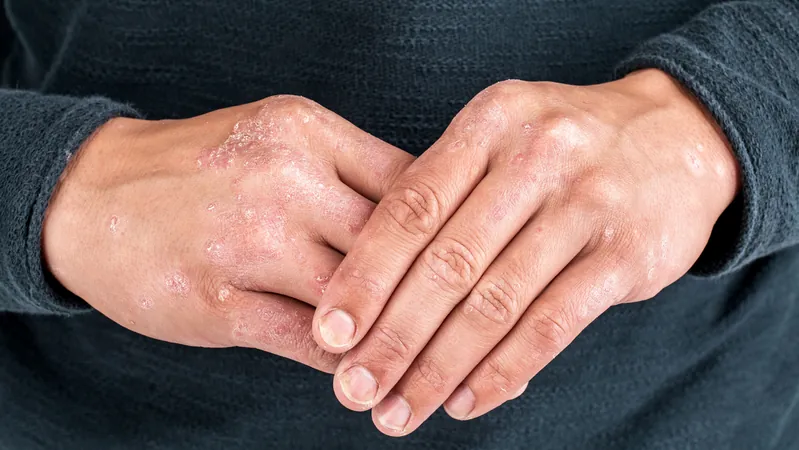
Breakthrough in Psoriasis Treatment: Artax Wins Clinical Validation for AX-158!
2025-01-22
Author: Wei
Introduction
In an exciting development for psoriasis treatment, Artax Biopharma has announced the successful clinical validation of AX-158, a groundbreaking oral medication specifically designed for individuals suffering from mild to moderate plaque psoriasis. Results from the phase 2a trial confirm the earlier preclinical and phase 1 findings, showing this innovative therapy could be a game-changer in the autoimmune treatment landscape.
CEO Announcement
“I am thrilled to announce that our unique approach to Nck modulation has demonstrated a notable impact on both relevant biomarkers and clinical measurements, especially within the moderate patient population,” said CEO Rob Armstrong, conveying the company’s enthusiasm for this pioneering treatment.
Trial Details
The phase 2a trial was a randomized, double-blind, placebo-controlled study conducted across multiple centers in the UK. Thirty participants were administered either 10mg of AX-158 or a placebo once daily over 28 days, followed by an additional 30-day safety monitoring phase.
Results and Safety Profile
The results are promising—AX-158 exhibited a favorable safety profile, with no severe adverse events or infections reported. Participants only experienced mild to moderate side effects, which were resolved quickly and did not require any medical intervention. Impressively, there were no discontinuations from the treatment, further indicating its tolerability.
Mechanism of Action
AX-158 works as an oral small molecule immunomodulator that selectively modulates T cell responses and acts as a T cell receptor. This unique mechanism represents a breakthrough in psoriasis treatment, with potential applications as either a standalone therapy or in combination with other treatments.
Insights from Dr. James Krueger
Dr. James Krueger, Head of Laboratory for Investigative Dermatology at The Rockefeller University and a member of Artax’s Scientific Advisory Board, conducted a comprehensive analysis of psoriasis biomarkers during the trial. He observed statistically significant changes in disease and pathway-related genes, correlating with improvements seen in the Psoriasis Area Severity Index (PASI) scores.
Future Directions
“What’s particularly exhilarating about this novel mechanism of action is its potential to influence the underlying disease processes. I eagerly anticipate observing how these responses develop over a more extended treatment period in future studies,” Dr. Krueger stated.
Company's Vision
Artax plans to showcase these groundbreaking results at an upcoming scientific conference, and importantly, they are already looking ahead to further investigate the potential of Nck modulation in treating other autoimmune diseases, such as atopic dermatitis.
Conclusion
With commendable gratitude, Armstrong acknowledged the extensive efforts of investigative clinicians, their teams, and all participating patients. “There remains significant work to be done in the autoimmune space. With AX-158 and our growing portfolio of Nck modulators, we are dedicated to bridging the treatment gap for patients,” he concluded.
Final Thoughts
Keep an eye on this innovative therapy—AX-158 could redefine the future of psoriasis management and beyond!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)