Breakthrough in Nuclear Physics: Discovery of a New 'Magic' Proton Number!
2025-08-20
Author: Nur
A Groundbreaking Discovery in Quantum Science
Physicists have made a stunning revelation regarding the mysterious world of atomic nuclei. The first precise mass measurements of silicon-22, an extremely short-lived and proton-rich nucleus, have unveiled a new 'magic number' of 14 protons. This discovery not only enhances our understanding of nuclear structure but also may revolutionize how we comprehend the strong nuclear force and the processes involved in element formation.
What Are Magic Numbers?
In the realm of stable nuclei, it's common for the numbers of neutrons and protons to be roughly equal. As protons—positively charged—rise in number, more neutrons are required to counteract their repulsive forces. Typically, an isotope will become unstable if it has either too few or too many neutrons. Back in 1949, scientists Maria Goeppert Mayer and J Hans D Jensen introduced the concept of 'magic numbers'—specific counts of nucleons that result in unusually stable nuclei. These 'magic' numbers, including 2, 8, 20, 28, 50, 82, and 126 for neutrons, indicate a fully filled nuclear shell, leading to incredible stability. However, the magic numbers for exotic, short-lived isotopes remain largely a mystery.
The Quest for New Proton Magic Numbers
Recent research has hinted that neutron-rich nuclei may have magic numbers of 14, 16, 32, and 34. Now, the search turns to proton-rich variants. Yuan-Ming Xing, a leading physicist at the Institute for Modern Physics, emphasizes the thrill of exploring new magic numbers in these unexplored proton-rich territories, where protons exist in an unusual, loosely bound state ripe for research.
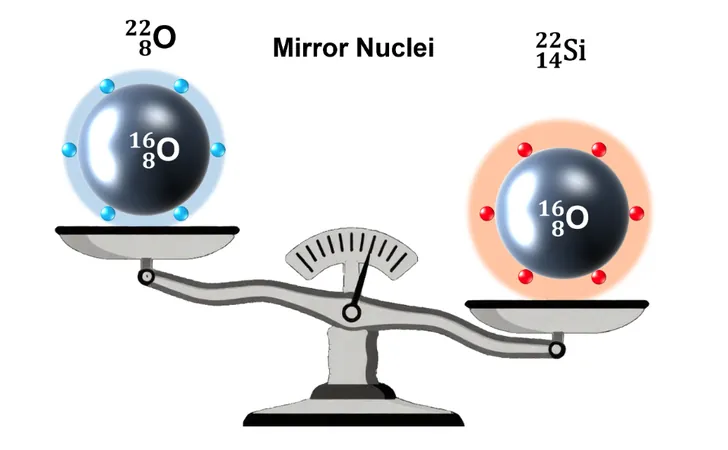
The Search for Mirror Nuclei
Drawing from fundamental nuclear theories, including isospin symmetry, scientists believe that mirror nuclei—nuclei with swapped quantities of protons and neutrons—should share the same magic numbers. After confirming that 14 is indeed a magic number for neutrons in oxygen-22 (8 protons, 14 neutrons), researchers sought its proton-rich counterpart: silicon-22 (14 protons, 8 neutrons).
Breaking Through Challenges in Research
Silicon-22's short half-life poses significant production challenges. To tackle this, researchers employed an enhanced B𝜌-defined isochronous mass spectroscopy technique. Operating in the Cooler-Storage Ring at China’s Heavy Ion Research Facility, they accelerated stable 36Ar ions and targeted them onto a beryllium source, generating silicon-22 nuclei. By measuring their movement through the storage ring, they confirmed magic number 14 for protons.
Implications for Understanding the Universe
This pivotal work could reshape our grasp of nucleon interactions and exotic nuclear structures, ultimately revealing the limits of existence for unstable nuclei. As team member Giacomo de Angelis from Italy's National Laboratories of Legnaro states, this finding opens the door for deeper investigations that may illuminate stellar element formation—crucial knowledge for astrophysicists modeling cosmic phenomena.
The Path Ahead: A Call for Further Research
De Angelis views this discovery as a powerful invitation for nuclear physicists worldwide to delve deeper. The upcoming High Intensity Heavy-Ion Accelerator Facility in Huizhou, China, is expected to enhance research capabilities, providing the tools to explore more loosely bound systems and unravel the enigmatic interactions governing our universe.
This thrilling breakthrough not only marks a pivotal moment in nuclear physics but also promises to unveil secrets that could transform our understanding of atomic interactions and the cosmic events that shape our existence.





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)