Breakthrough Discovery Unveils Pathway for Targeted Therapies in Blood Cancers Linked to TET2 Gene Mutation
2024-11-03
Author: Daniel
Breakthrough Discovery Unveils Pathway for Targeted Therapies in Blood Cancers Linked to TET2 Gene Mutation
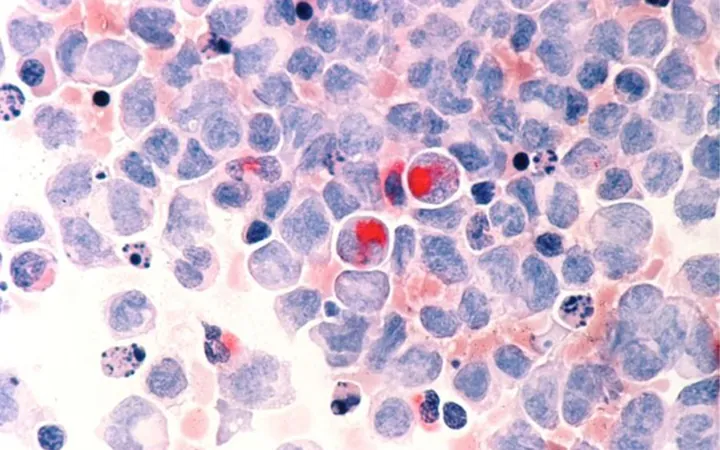
In an exciting development for the field of hematology, approximately 30% of individuals with myeloid malignancies have a mutation in the gene known as tet methylcytosine dioxygenase 2 (TET2). This crucial gene is integral in regulating protein creation and has demonstrated tumor-suppressor properties.
A ground-breaking study, published in September's issue of Nature, marks the first time researchers have unraveled the enzymatic activity pathway of TET2, essential to its tumor-suppressing function. This collaborative research initiative involved scientists from UT Health San Antonio and the University of Chicago, including co-primary investigators Mingjiang Xu from UT and Chuan He from Chicago.
This discovery paves the way for targeted treatments and preventative measures for cancers associated with TET2 mutations, Xu commented, emphasizing the significance of their findings.
The implications of TET2 mutations have been evident for years, with links established to various blood cancers such as chronic myelomonocytic leukemia, acute myeloid leukemia, and myelodysplastic syndromes. However, the underlying mechanisms through which these mutations contribute to disease progression have remained elusive—until now.
Xu is no stranger to the TET2 mutation saga, being one of the first researchers to demonstrate its causative role in myeloid malignancies using mouse models around 15 years ago. His pioneering work sparked a global research effort aimed at unveiling the complexities surrounding TET2's function. Unfortunately, many researchers encountered hurdles by only focusing on its impact on DNA, missing other crucial aspects.
Shifting the Focus: Understanding RNA's Role
Under Xu's guidance, his team made a decisive turn in research direction. Recognizing that TET2’s influence on DNA was insufficient to explain its tumor-suppressive capabilities, they hypothesized that TET2's enzymatic activities might involve RNA—a more intricate facet of gene expression modulation.
By partnering with RNA modification expert Chuan He, the team discovered that TET2 could modify chromatin-associated RNA, resulting in significant alterations in gene expression conducive to tumor suppression. This breakthrough highlighted promising new therapeutic targets in the fight against blood cancers.
We were astounded by the profound impact this pathway has on TET2’s role in regulating gene expression, Xu noted. The study revealed that the absence of TET2 creates a permissive chromatin environment, enabling gene expression changes that could trigger the uncontrolled proliferation of blood cancer cells.
One promising novel target identified is the reader protein MBD6, which recognizes specific RNA modifications and could serve as an effective therapeutic intervention for MYELOID malignancies associated with TET2 mutations.
Towards Targeted Therapies
Xu expressed his optimism that the findings could revolutionize the landscape of treatments for blood cancers linked to TET2 mutations. Future research will aim to create molecular inhibitors that can disrupt this newly identified pathway, leading to innovative targeted therapies.
Apart from its role in blood cancers, research indicates that around 10% of individuals aged 70 and older harbor certain genetic mutations, including TET2 mutations, which could lead to clonal hematopoiesis of indeterminate potential (CHIP). Xu highlighted the urgency of these findings, stating that such individuals face significantly heightened risks of developing both myeloid cancers and cardiovascular diseases.
The growing aging population necessitates intervention strategies, Xu cautioned, underscoring the critical need for preventive measures in managing these health risks.
This study represents a significant milestone in Xu’s career, marking a culmination of years devoted to researching TET2 and RNA modifications. His collaboration with UT Health San Antonio and the University of Chicago illustrates the power of teamwork in unraveling complex medical mysteries and advancing therapeutic options for patients affected by these devastating diseases.
Stay tuned as this exciting field of research unfolds! The journey to targeted blood cancer therapies may be closer than we ever imagined.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)