Breakthrough Discovery: How RAD52 Protein Could Revolutionize Cancer Treatment
2025-04-02
Author: John Tan
Breakthrough Discovery: How RAD52 Protein Could Revolutionize Cancer Treatment
In a groundbreaking study led by researchers at the University of Iowa, scientists have unveiled a surprising new structure of the DNA repair protein RAD52. This discovery could pave the way for the development of innovative cancer therapies targeting cells with faulty DNA repair mechanisms.
The study, recently published in the prestigious journal Nature, highlights the critical role of RAD52 in safeguarding replicating DNA within dividing cells. According to Dr. Maria Spies, a professor of biochemistry and molecular biology, RAD52 is a highly sought-after target for drugs that could treat cancers characterized by DNA repair deficiencies, such as breast, ovarian, and certain types of glioblastomas.
"RAD52 is essential for the survival of cancer cells that lack the ability to repair DNA, unlike healthy human cells where this protein is not critical," Spies explained. The dependency of some cancers on RAD52 opens up a unique window of opportunity for therapies that inhibit this protein, potentially leading to the selective destruction of malignant cells while sparing healthy tissues.
Research shows that inhibiting RAD52 could be as effective as using PARP inhibitors, a class of drugs already in clinical use that targets cancers with BRCA1 and BRCA2 mutations. While these PARP inhibitors help around 15% of patients live disease-free for over five years, many quickly develop resistance. Therefore, developing drugs that target RAD52 in conjunction with or independent of PARP inhibition could significantly broaden the range of treatment options.
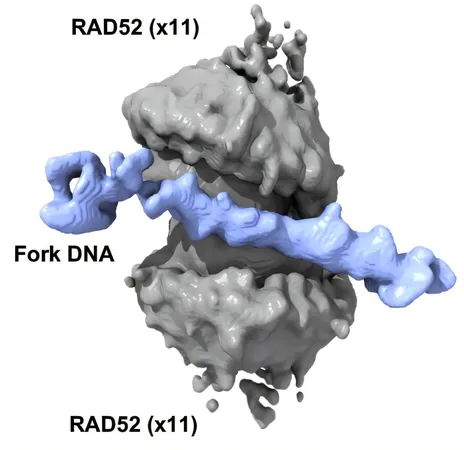
Visualizing RAD52's Double Ring Structure
The research team employed advanced cryogenic electron microscopy (CryoEM) to illuminate the previously unknown double-ring configuration of RAD52. This structure consists of two rings—each made up of 11 RAD52 proteins—engaging various components of DNA at critical sites known as replication forks. This arrangement not only assists in protecting the DNA from degradation but also rearranges the DNA structure for enhanced stability.
Dr. Pichierri from the Istituto Superiore di Sanità in Rome collaborated with Spies and her team to shed light on this innovation, indicating that the ability of RAD52 to support stalled DNA replication forks is vital for the resilience of cancer cells.
"This novel spool-like structure reveals potential targets for drug therapy, marking a significant advancement in our understanding of how RAD52 operates at a molecular level," Spies said.
A Promising Future for Targeted Cancer Therapies
The implications of this structural understanding are immense. Researchers already possess small molecules that can bind and inhibit RAD52, but further refinement is essential to enhance their specificity and efficacy. The combined expertise of the researchers from Iowa and Rome—employing computational modeling, cell imaging, and super-resolution techniques—has provided critical insights into how RAD52 functions as a gatekeeper during DNA replication.
As cancer researchers continue to explore this promising avenue, the hope is to transition from understanding the structure and function of RAD52 to creating effective inhibitors that can improve patient outcomes. With cancer treatments often fraught with severe side effects, the potential for therapies that selectively target RAD52 while minimizing harm to healthy cells is a ray of hope for those battling cancer.
This exciting study not only holds promise for current cancer therapies but also lays the groundwork for future research into the RAD52 protein's roles and regulatory mechanisms, potentially harnessing it as a powerful weapon in the fight against cancer. Keep an eye out—new anti-cancer drugs could soon change the landscape of treatment options available to patients!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)