Unleashing the Power of Microwave Pulses: The Future of Ion-Molecule Reactions at Near Absolute Zero!
2025-04-03
Author: Daniel
In a groundbreaking revelation in the field of molecular physics, researchers at ETH Zurich have made significant strides in controlling chemical reactions at extremely low temperatures—below 10 K, to be precise. This breakthrough could reshape our understanding of chemistry in environments akin to the vast, frigid interstellar clouds where such reactions are pivotal.
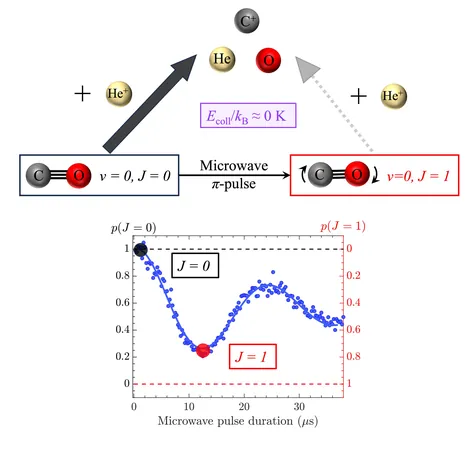
The core of their research focuses on the unique behavior of ion-molecule reactions at these low temperatures, where the speed of chemical reactions is intricately tied to the rotational states of the molecules involved. Researchers Valentina Zhelyazkova, Fernanda B. V. Martins, Frédéric Merkt, and their team have unveiled a novel approach that utilizes microwave pulses—electromagnetic waves with frequencies in the range of 300 MHz to 300 GHz—to manipulate these molecular rotational states.
Zhelyazkova remarked, “Our research has demonstrated that reactivity can vary dramatically based on the rotational state of the molecules. We have been able to study these reactions in conditions that mirror what might exist in the depths of space.”
The researchers intentionally cooled the target molecules to their rotational ground state, where they are known to be most reactive. By applying precisely timed microwave pulses, they could transition the molecules to a higher rotational state, thereby reducing their reactivity. This was evident as they monitored the rate of product formation in response to the microwave-induced changes.
"The key finding was that we could slow down the reaction rate without thermal heating, which is contrary to traditional methods that rely on increasing temperature to enhance reactivity. Our method opens a door to manipulate chemical processes fundamentally differently," explained Martins.
Essentially, this work demonstrates that the rotational state of a molecule is not just a passive characteristic but a dynamic factor that can be manipulated to control chemical reactions. The implications of this study could significantly impact various fields, from astrochemistry—where many of these reactions are believed to occur naturally—in the cosmos to practical applications in chemical synthesis right here on Earth.
Looking ahead, the research team aims to refine their microwave-based techniques further. According to Merkt, “Our next steps involve creating molecules in targeted nonreactive quantum states and using microwave pulses as a method to trigger specific reactions. This could lead to more controlled and efficient chemical syntheses.”
As we stand on the brink of a new era in molecular chemistry, this innovative approach could revolutionize how we manipulate chemical processes, possibly leading to groundbreaking advancements in technology and our understanding of the universe. Keep an eye on these researchers as they continue to push the boundaries of science!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)