Breakthrough Discovery Could Revolutionize Biomaterial Implants and Combat Fibrosis
2024-10-24
Author: Sarah
A groundbreaking study conducted by the University of Virginia’s Department of Biomedical Engineering has revealed a significant mechanism behind fibrosis, more commonly known as scarring, linked to medical implants made from biomaterial hydrogels. Published in the June 14 edition of "Science Advances," the research offers fresh optimism for improving the durability and effectiveness of various implantable devices, including sensors, stents, and drug delivery systems.
Unexpected Findings from Microporous Hydrogels
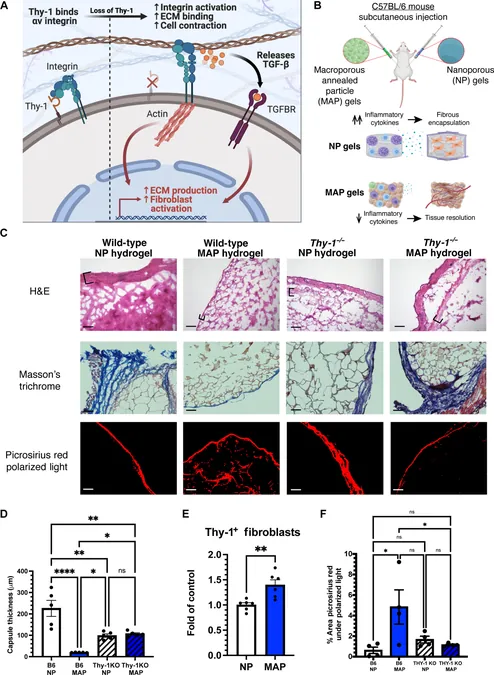
In their research, the scientists explored an advanced class of materials known as microporous annealed particle (MAP) hydrogels, co-invented by Griffin. Typically, these hydrogels integrate seamlessly with the body, avoiding scar formation. Yet, the study discovered that when the essential protein Thy-1 was absent in mice, even these advanced materials sparked scar tissue formation.
Previously, Barker's research indicated that Thy-1 functions as a regulatory "brake" that limits fibroblast activity, preventing excessive scar tissue formation. However, the precise cause of Thy-1 loss in the context of implanted materials remained elusive.
Unraveling the Inflammation-Fibrosis Connection
Suspecting inflammation to be the culprit, the researchers subjected fibroblasts to inflammatory proteins and noted that only a unique subset lost the Thy-1 marker. “Interestingly, these cells exhibited immune-related gene expressions,” Abebayehu explained. “We have documented similar 'inflammatory fibroblasts' in conditions such as cancer and liver cirrhosis, but their presence in the vicinity of biomaterials was unexpected.”
Understanding this connection between Thy-1 negative cells and fibrosis sheds light on a complex web of fibrotic diseases, which currently lack effective treatments. Abebayehu stated, “Chronic inflammation and fibrosis are correlated, but the mechanism behind their relationship is still unclear.”
Their findings propose that persistent inflammatory conditions might trigger the emergence of these specific fibroblasts, which mistakenly perceive tissue stiffness and amplify the scarring processes. “These Thy-1 negative 'inflammatory fibroblasts' could bridge the gap between chronic inflammation and fibrosis, potentially explaining why anti-inflammatory treatments have failed in some cases,” Abebayehu said.
Transforming Medical Outcomes
Targeting these fibroblasts or the signaling pathways that influence their development could substantially reduce implant failures for countless patients requiring heart devices and joint replacements. Barker emphasized the broader implications, noting, “Fibrosis is responsible for nearly half of the deaths in developed nations. Gaining insights into how fibrosis initiates and persists could guide us toward the development of effective treatments.”
Future Directions in Research
The research team plans to investigate innovative designs for biomaterials that encourage Thy-1 presence in fibroblasts. Their goal is to transform implants that currently foster fibrosis into non-fibrotic alternatives, akin to Griffin’s MAP hydrogels, which not only minimize scarring but also support the healing and regeneration of healthy tissue.
Additionally, they aim to delineate the various cell types and their distinct “flavors” that appear around biomaterials causing fibrosis. Abebayehu stated, “This will enable us to uncover how inflammatory fibroblasts emerge, understand their communication networks, and ultimately detail how they contribute to the onset of full-scale fibrosis.”
This research stands on the cusp of potentially revolutionizing the field of biomedical implants, heralding a new era in patient care where complications might be drastically minimized. Stay tuned for further updates as their findings could reshape the future of medical implant technology.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)