Revolutionary Breakthrough: Scientists Capture a Single Electron in Motion During a Chemical Reaction!
2025-08-29
Author: Ken Lee
In an unprecedented achievement, scientists have successfully captured the movement of a single electron during a chemical reaction using ultrafast X-ray flashes. This groundbreaking discovery marks the first time this has been done, opening up new avenues in the field of chemistry and materials science.
Published on August 20 in the journal *Physical Review Letters*, the study showcases the researchers' ability to image a valence electron—the outermost electron in an atom—while an ammonia molecule disintegrated. This achievement defies decades of research limitations, where previous methods primarily focused on core electrons.
For years, ultrafast X-ray scattering techniques have allowed scientists to freeze molecular movements in time. However, they struggled to study valence electrons, the real players in chemical reactions. Ian Gabalski, a physics doctoral student and lead author, expressed the team's goal: "We wanted to take pictures of the actual electrons that are driving that motion." Understanding this electron movement could revolutionize drug design, improve chemical processes, and enhance material efficiency.
The choice of ammonia as the subject molecule was strategic. Gabalski noted, "Ammonia is special because it consists mostly of light atoms, minimizing interference from core electrons and allowing us to better observe the valence electron."
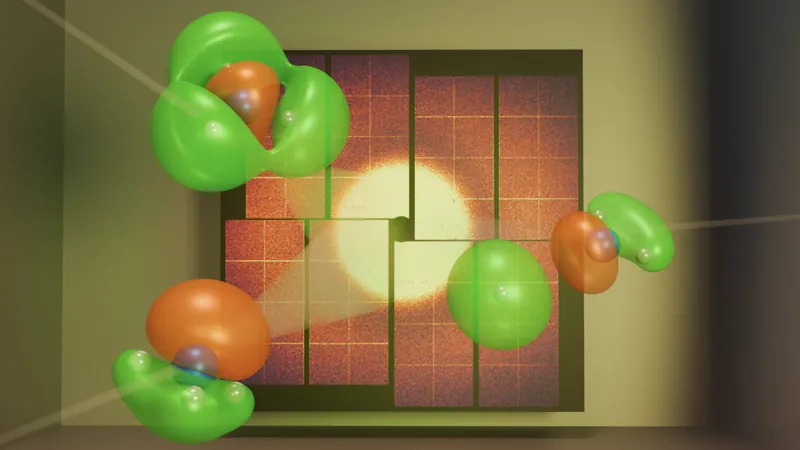
Conducted at the SLAC National Accelerator Laboratory's Linac Coherent Light Source, the experiment began by exciting the ammonia molecule with a burst of ultraviolet light. This energy surge caused one of its electrons to leap to a higher energy state, triggering a cascade of chemical reactions. With the intense X-ray beam, the researchers meticulously tracked how the electron's "cloud"—a quantum probability representation of its location—altered as the molecule began to break apart.
In quantum physics, electrons resemble more of a diffuse cloud rather than a solid entity. Gabalski explains that this cloud reflects where the electron is most likely to be found, with varying densities indicating potential spots. The team utilized quantum mechanical simulations to map the electronic structure of the ammonia molecule, painting a clearer picture of the electron’s dynamics.
As the X-rays penetrated this electron cloud, they scattered in distinct patterns, which the researchers expertly analyzed to reconstruct an image of the electron's movement during the reaction. Their findings validated a theoretical model that incorporated valence electron motion, confirming the successful observation of the electron's rearrangement.
Looking ahead, the researchers aim to adapt their groundbreaking system for complex, 3D environments, mimicking real-life tissues. This innovation could pave the way for remarkable advancements in regenerative medicine, potentially enabling the on-demand growth or repair of tissue!
This pioneering work not only pushes the boundaries of scientific understanding but also holds extraordinary implications for the future of material science and medicine, marking a new chapter in the exploration of the atomic world.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)