Unlocking the Secrets of Star Formation: How Molecules Shaped the Universe
2025-07-29
Author: Charlotte
The Cosmic Dawn After the Big Bang
Picture the universe moments after the Big Bang—around 13.8 billion years ago. It was a tempestuous realm of extreme temperatures and densities. Within seconds of this cataclysmic event, things began to cool, allowing for the formation of the universe's first elements: hydrogen and helium. But these elements remained completely ionized until nearly 380,000 years later when the universe cooled enough for neutral atoms to emerge, setting the stage for the first chemical reactions.
From Helium Hydride to Hydrogen: The Birth of Molecules
The very first molecule to exist was the helium hydride ion (HeH⁺), a product of a neutral helium atom meeting an ionized hydrogen nucleus. This pivotal moment launched a chain reaction that resulted in the creation of molecular hydrogen (H₂), the most abundant molecule in the cosmos.
The Cosmic Dark Ages: Waiting for Stars
Despite the formation of neutral atoms, the universe soon plunged into what astronomers call the "dark age". With no stars or light-emitting objects in sight, the universe was transparent but devoid of warmth and glow. It took several hundred million years for the first stars to finally ignite.
Molecules as the Builders of Stars
During these ancient epochs, simple molecules like HeH⁺ and H₂ played vital roles in star formation. For a gas cloud to collapse into a protostar capable of nuclear fusion, it must shed heat. This cooling arises from molecular collisions, which excite atoms that subsequently release energy in the form of photons.
A Cooling Agent's Role: The Power of HeH⁺
As the temperature dropped below about 10,000 degrees Celsius, hydrogen's ability to cool became less effective. Cooling could only continue through molecules that emitted energy via rotational and vibrational movements. The HeH⁺ ion, known for its robust dipole moment, emerged as a crucial player in cooling the primordial environment and facilitating early star formation.
Unveiling Ancient Reactions in the Lab
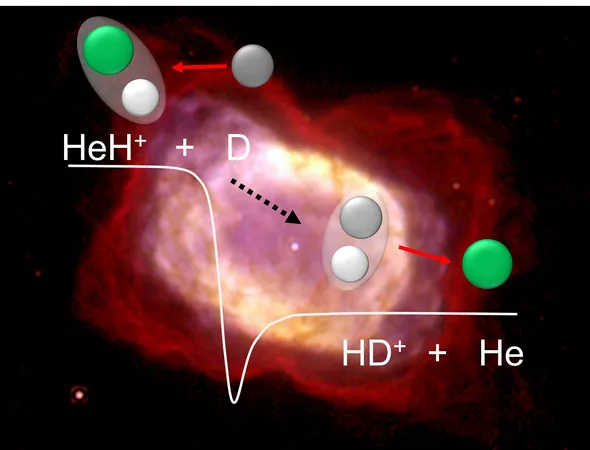
Exciting new research from the Max-Planck Institute for Nuclear Physics (MPIK) in Heidelberg has managed to replicate the early universe's conditions and examine these ancient reactions. Scientists studied how HeH⁺ reacts with deuterium, a heavier hydrogen isotope. This reaction produces HD⁺ instead of H₂⁺, alongside a neutral helium atom.
Pioneering Experiments at Cryogenic Temperatures
Conducted at the one-of-a-kind Cryogenic Storage Ring (CSR) at MPIK, the team explored molecular interactions under space-like conditions. In this setup, HeH⁺ ions were stored for up to 60 seconds at frigid temperatures near absolute zero, colliding with a beam of neutral deuterium atoms. By adjusting the collision speeds, researchers observed how reaction rates varied with temperature.
Challenging Prevailing Theories
Surprisingly, the reactions did not slow down at lower temperatures as previously theorized; they remained constant instead. Dr. Holger Kreckel from MPIK stated, "Previous theories predicted a significant decrease in reaction probability at low temperatures, but our findings challenge that notion." This suggests that interactions involving HeH⁺ were far more significant in the chemistry of the early universe than scientists once thought.
New Insights for the Future
This groundbreaking work aligns with recent findings by theorists led by Yohann Scribano, who uncovered errors in prior calculations regarding reaction potentials. With improved models corresponding to CSR's experimental results, our understanding of cosmic chemistry is evolving, shedding light on how those first stars were born.









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)