Unlocking the Secrets of Mitochondrial Repair: Scientists Discover a Revolutionary Recycling System
2025-04-07
Author: William
Recent research has unveiled a groundbreaking mechanism that helps protect and repair mitochondria, the powerhouse of our cells. Damage to mitochondrial DNA (mtDNA) can have severe consequences, linking it to serious conditions such as Parkinson's disease, Alzheimer's disease, amyotrophic lateral sclerosis (ALS), cardiovascular issues, and type 2 diabetes. Moreover, mtDNA damage accelerates the aging process, making cellular health paramount in the fight against these diseases.
A collaborative study by scientists from University Hospital Düsseldorf, Heinrich Heine University (HHU), the University of Cologne, and the Center for Molecular Medicine Cologne (CMMC) has shed light on a specialized recycling system that cells employ when mtDNA damage is detected. Led by Professor Pla-Martín from the Institute of Biochemistry and Molecular Biology I at HHU, the research team reveals the intricate workings of this cellular mechanism in a recent issue of Science Advances.
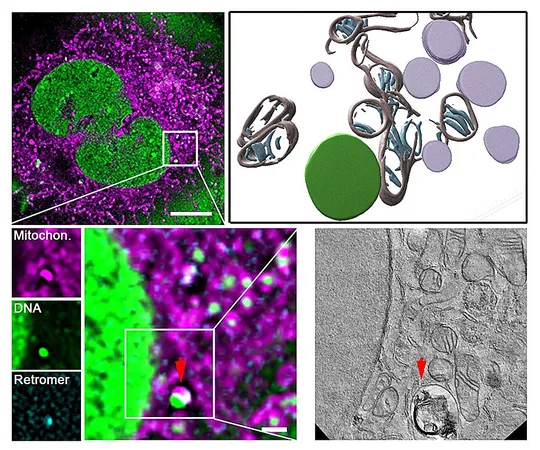
At the heart of this discovery is a protein complex known as the retromer, which works alongside lysosomes—cell organelles packed with digestive enzymes that function like recycling centers. This unique system identifies faulty mtDNA and facilitates its removal, preventing the accumulation of damaged genetic material and ensuring cellular vitality.
Professor Pla-Martín emphasized the significance of this finding: “We have identified a previously unknown cellular pathway crucial for mitochondrial health and, consequently, the natural defenses of our cells. Understanding this mechanism could reveal how mitochondrial damage instigates diseases like Parkinson's and Alzheimer's, providing a new avenue for developing preventive therapies.”
Remarkably, this research built on the expertise of Dr. Parisa Kakanj from the University of Cologne, who employed fruit flies (Drosophila) as a model organism to validate and expand the findings. Her work demonstrated that accelerating the activity of the retromer complex—particularly the protein VPS35—significantly enhances the rate at which damaged mtDNA is eliminated, subsequently improving mitochondrial function.
Dr. Kakanj noted, “Using Drosophila allowed us to confirm our initial findings in human cells and show marked improvements in mitochondrial health. This opens up exciting possibilities for developing targeted therapeutic strategies against mitochondrial diseases and age-related conditions.”
This groundbreaking research not only deepens our understanding of mitochondrial repair mechanisms but also raises hopes for innovative treatments that could combat diseases linked to mitochondrial dysfunction. As our understanding of cellular repair systems grows, the prospect of effective preventive therapies could soon become a reality, sparking new hope for millions affected by these debilitating conditions. Stay tuned for more developments in this exciting field of medical research!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)