Revolutionary Green Synthesis Technique Unlocks New Potential for Semiconductor Photocatalysis in Hydrogen Production!
2024-11-07
Author: Michael
Introduction
Solar-powered photocatalytic water splitting is emerging as a groundbreaking method for sustainable hydrogen production, yet it faces significant challenges. While researchers have investigated various semiconductor materials, persistent issues such as bandgap limitations and carrier recombination continue to impede progress.
Emergence of CdNCN
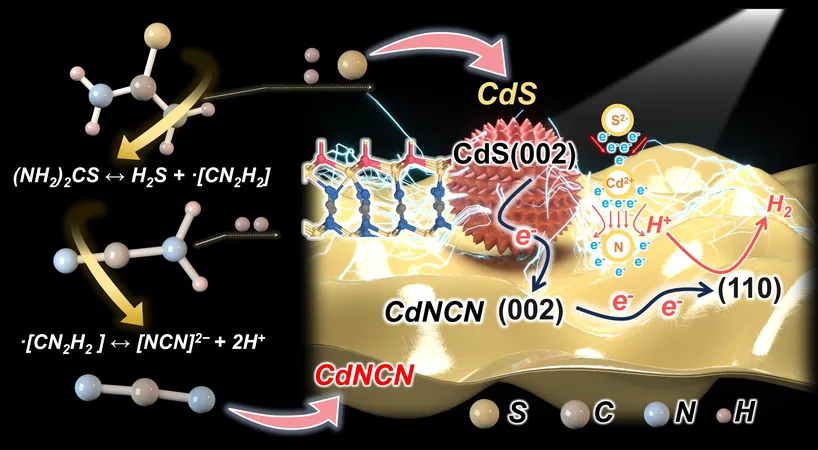
Enter CdNCN, a transition metal carbodiimide that is garnering attention for its advantageous bandgap and robust covalent bonding properties. By integrating CdNCN with CdS heterostructures, researchers aim to enhance electron transport and separation mechanisms through sophisticated quasi-crystalline transition sites. However, the traditional synthesis of CdNCN has been marred by reliance on toxic reagents, which raises serious concerns regarding environmental impact and scalability.
A Breakthrough in Synthesis
Recently published in *Advanced Powder Materials*, a collaborative study involving researchers from China and Australia introduces an innovative synthesis strategy for creating CdNCN-CdS heterostructures under benign environmental conditions. This breakthrough promises to pave the way for more efficient solar-driven photocatalytic hydrogen production processes.
One-Pot Synthesis Method
A pivotal aspect of the study is the development of a “one-pot” synthesis method employing thiourea. This technique streamlines the overall process by generating [NCN]2- moieties during its decomposition phase. Senior researcher Shengsen Zhang stated, “By leveraging the electron-attracting properties of the carbodiimide group in CdNCN, we have established a rapid pathway for electron transfer, achieving record-breaking hydrogen evolution efficiency without the need for additional cocatalysts.”
Record-Breaking Results
The results of their work are impressive: the optimized CdNCN-CdS heterostructure achieved a hydrogen evolution rate of 14.7 mmol g-1 h-1 under visible light, shattering records set by previous CdS-based catalysts. Zhang elaborated, “This exceptional performance is primarily due to the formation of atomic-level N–Cd–S transition sites, which significantly reduce electron transfer resistance and channel electrons directly to the CdNCN (110) plane—the ideal site for hydrogen adsorption.”
Precision in Catalyst Composition
One remarkable feature of this research is the precision in controlling catalyst composition through adjustments to the Cd-to-thiourea ratio. The team employed in-situ spectroscopy techniques to demonstrate that intermediate [NCN]2- moieties form strong bonds with Cd atoms, effectively enhancing charge transport across the heterostructure.
Conclusion
This cutting-edge research not only addresses the challenges of semiconductor-based photocatalysis but also exemplifies the importance of adopting green and scalable synthesis methods. This critical step brings us closer to achieving sustainable hydrogen production—an endeavor that could reshape the future of clean energy and address global energy demands. The innovative approach could potentially revolutionize the industry, making hydrogen not just a dream, but a powerful and clean alternative for energy storage and transportation. Stay tuned for more updates as researchers continue to unveil the exciting possibilities of semiconductor photocatalysis!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)