Revolutionary Fluorescence Technique Unveils Supercharged Glycan-Cleaving Enzyme
2025-09-23
Author: William
Unlocking the Secrets of Glycans
Glycans, intricate sugar chains attached to proteins, significantly influence how these proteins operate. The ability to efficiently remove glycans can greatly enhance our understanding of both the proteins and their sugar counterparts. However, the challenge remains: many glycans lack effective enzymes for their removal, particularly O-glycans, which adorn about 83% of proteins released by cells.
A Breakthrough in Enzyme Engineering
In a groundbreaking study spearheaded by Stephen Withers at the University of British Columbia, researchers have engineered a remarkably potent enzyme capable of cleaving O-glycans. This newly developed enzyme boasts an astounding 840-fold enhancement in cleaving the elusive sialyl T-antigen (STAg) compared to its natural counterpart.
Innovative Genetic Modifications
To create this enzyme, Withers and his team took an existing enzyme with minimal STAg-cleaving capability and modified it. They introduced random mutations in the gene sequence within *Escherichia coli* cells, particularly targeting the DNA that codes for the enzyme's active site. This ‘directed evolution’ approach generated a vast library of E. coli cells, hoping that among the tens of thousands, one would exhibit exceptional cleaving efficiency.
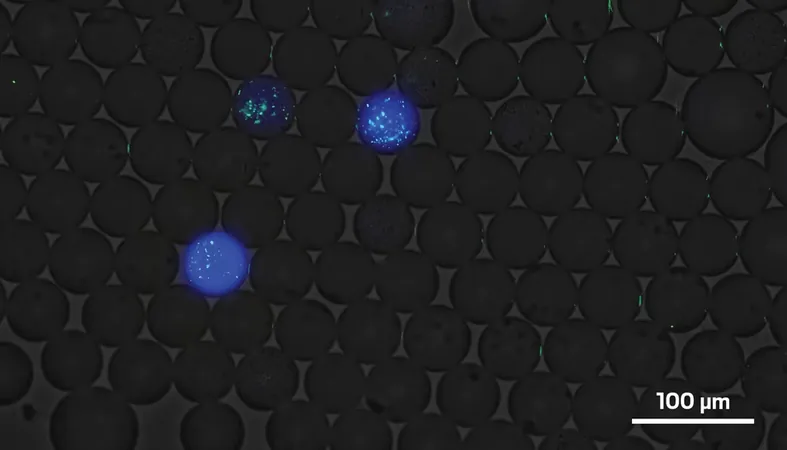
A Fluorescent Screening Method Like No Other
The researchers utilized a novel screening technique incorporating fluorescent markers. By suspending oil droplets containing individual E. coli cells in water, they added proteins attached with STAg glycans and DNA that emitted blue fluorescence upon removal of the glycan. After a few days, they observed the glowing droplets to measure enzyme activity.
What set this study apart was the additional introduction of DNA that caused the enzymes themselves to fluoresce green. By analyzing the brightness of both the blue and green emissions, researchers could discern which enzymes were particularly effective at cleaving STAg, even if they weren't expressed abundantly in E. coli. This crucial step allowed them to thoroughly evaluate and potentially discover powerful enzymes that could have been overlooked.
Expert Praise and Future Implications
Stacy Malaker, a chemist at Yale School of Medicine and an outside observer of the study, remarked, "The methodology they’ve developed is really unique. I’ve never seen anything like it." With this innovative approach, Withers’ team achieved an enzyme that could cleave STAg 840 times more efficiently than the original enzyme, which vastly exceeded their expectations. While typical enhancements yield only a 5-10 fold increase, this remarkable leap underscores the promise of their new method.
A Bright Future for Glycan Research
Malaker further highlights the transformative potential of this research, noting that it lays the foundation for engineering enzymes capable of targeting biologically significant or disease-related O-glycans. This has exciting therapeutic implications, such as selectively removing glycans exclusive to tumor cells. Withers suggests that the fluorescent technique could be adapted for broader applications, allowing scientists to optimize various enzymes across different biological reactions. The future of glycan research has truly never looked brighter!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)