Revealing the Shocking Truth: Forever Chemicals are Even More Acidic than We Thought!
2025-09-04
Author: Benjamin
Groundbreaking Discovery about Forever Chemicals
BUFFALO, N.Y. — In a stunning revelation, scientists have uncovered that per- and polyfluoroalkyl substances (PFAS), notorious for their persistence in the environment, are far more acidic than previously believed. This finding significantly alters our understanding of how these 'forever chemicals' behave in nature.
Why Acidity Matters
PFAS are known for their toxic impact, and their high acidity allows them to easily shed protons, making them negatively charged and enabling them to dissolve and spread through water more readily. This new research out of the University at Buffalo highlights how this increased acidity affects both environmental mobility and human health risks.
New Method Unveils Hidden Acidic Potential
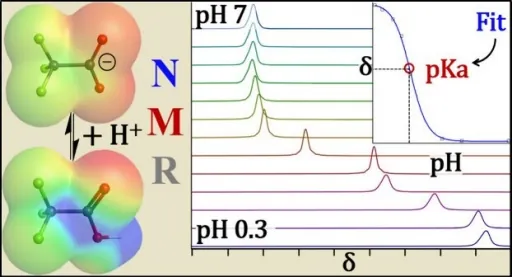
The research team, led by a senior scientist from the UB RENEW Institute, employed an innovative experimental approach to measure the acidity of 10 types of PFAS and three common breakdown products. Their findings revealed that the acid dissociation constants, or pKa values, were often dramatically lower than previously documented, with GenX, a Teflon substitute, being found to have a pKa about one thousand times lower than earlier studies reported.
Implications of the Findings
"These findings suggest that previous measurements have seriously underestimated PAMFAS acidity, misrepresenting their persistence and spread in the environment," explained Alexander Hoepker, the corresponding author of the study. Accurate pKa measurements are vital for understanding whether these chemicals remain dissolved in water, cling to soil, or even vaporize into the air.
Innovative Techniques Enhance Accuracy
The UB research team tackled longstanding disagreements regarding pKa measurements by utilizing advanced techniques, including fluorine and proton nuclear magnetic resonance (NMR) spectroscopy. This allowed them to accurately determine whether a PFAS molecule is charged or neutral, an essential factor in calculating acidity.
The Challenge with Traditional Methods
Historically, PFAS has been tricky to measure due to their tendency to stick to glass, which skewed previous bulk measurements. The UB team's approach not only minimized these pitfalls but also integrated computational analyses to refine their findings further.
A Closer Look at Specific PFAS
Their studies specifically revisited hazardous PFAS like perfluorooctanoic acid (PFOA) and trifluoroacetic acid (TFA), revealing PFOA's pKa to be −0.27—meaning it’s highly likely to be charged under normal conditions, contradicting previous values that reached as high as 3.8. TFA was found to be significantly more acidic with a pKa of around 0.03.
The Future of PFAS Research
The implications of this research are profound, paving the way for better risk assessments, remediation technologies, and analytical methods for emerging PFAS contaminants. This improved understanding of acidity will help scientists tackle the ongoing environmental challenges posed by these dangerous chemicals.
Collaboration and Support Behind the Research
The groundbreaking study was supported by the National Science Foundation and involved collaboration with experts from St. Bonaventure University and Spain’s Institute of Environmental Assessment and Water Research.
As we continue to grapple with the implications of PFAS in our environment, this new research brings hope for more effective measures to mitigate their effects.






 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)