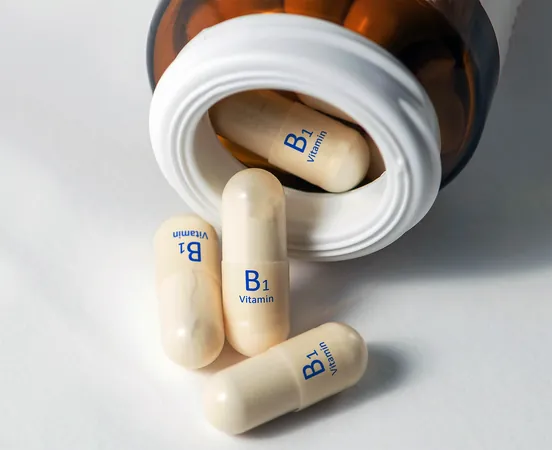
Groundbreaking Discovery: Vitamin B1's 1958 Carbene Theory Finally Validated!
2025-08-30
Author: Amelia
A Chemistry Breakthrough 65 Years in the Making
For decades, students have been taught a fundamental rule in chemistry: high-energy carbon species like vitamin B1 break down almost instantly in water. This long-held belief led many reactions to be carried out in specialized organic solvents instead of our most abundant resource—water. But a recent study challenges this notion, revealing that reactive carbon species can actually last long enough in water to be observed and described.
The 1958 Debate: A Bold Idea
The discussion around vitamin B1, also known as thiamine, ignited back in 1958 when researchers proposed a daring theory: that it could form a brief, carbene-like state in cells. Such a claim flew in the face of established thought that water would instantly dismantle carbenes. Despite the backlash, the pursuit of evidence persisted as technological advancements offered new tools to chemists.
The Breakthrough Evidence Has Arrived!
Fast forward to today, and scientists have finally cracked the code! They successfully designed a molecule that encapsulates the reactive carbon center, allowing it to withstand the aqueous environment long enough to be detected. Professor Vincent Lavallo from UC Riverside noted, “This is the first time anyone has been able to observe a stable carbene in water,” effectively silencing skeptics who deemed the theory outrageous.
Understanding Carbenes and Their Chemistry
So, what exactly is a carbene? It's a carbon atom that has two unfilled bonding spots, making it highly reactive. While this reactivity is advantageous for many lab and industrial reactions, it led chemists to previously dismiss their existence in watery settings due to quick reactions with water molecules. The new study outsmarts that limitation.
How the Discovery Was Made
Researchers employed a brilliant method to shield the carbene by surrounding it with bulky groups, effectively reducing unwanted side reactions while keeping the carbon center active. They used nuclear magnetic resonance (NMR) spectroscopy to capture its unique signature, subsequently cementing their findings with a detailed X-ray structure.
The Link to Vitamin B1 and Future Implications
Vitamin B1 plays a pivotal role as a cofactor in metabolism, enabling crucial carbon-bond transformations. This research provides the first tangible evidence supporting the 1958 hypothesis that carbenes can exist long enough to impact these fundamental processes. Moreover, it opens the door to safer chemical manufacturing practices. As Varun Raviprolu, the study's lead author, stated, “If we can harness these powerful catalysts in water, it’s a major leap toward greener chemistry.”
Looking Ahead: The Future of Carbene Chemistry
This breakthrough doesn't just stop here; it paves the way for isolating other elusive reactive intermediates. Lavallo expressed optimism, stating, “With strategies like ours, we may finally observe and learn from these short-lived species.”
Why This Matters
Although the study doesn't present a video of vitamin B1 forming a carbene within living cells, it fundamentally shifts the narrative. It demonstrates that water isn't a deterrent for carbene chemistry, reinforcing Brenlow's theory from 1958 and offering insights into conducting powerful reactions in the safest solvent available.
As Raviprolu concluded, "It turns out our work ended up confirming exactly what Breslow proposed all those years ago." This remarkable journey from theory to empirical evidence serves as a testament to the enduring power of scientific inquiry.






 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)