Breakthrough in Photocatalytic Water Splitting: New Catalyst Minimizes Charge Recombination!
2024-11-04
Author: Jacob
Introduction
A groundbreaking discovery by a team of researchers has the potential to revolutionize photocatalytic water splitting, a process hailed as the "Holy Grail" of chemistry for hydrogen production. Published in the prestigious journal *Nature Chemistry*, this study introduces a cutting-edge metal-organic framework (MOF) that effectively suppresses charge recombination, a significant barrier to achieving efficient hydrogen production.
Understanding Charge Recombination
Traditional methods to inhibit charge recombination primarily focus on the catalyst’s ground-state structure. However, this research explores the dynamics of what happens during the excited state—the phase when electrons and holes may recombine and diminish hydrogen production efficiency. By drawing inspiration from natural photosynthesis, where proteins change structure to facilitate electron transfer, the research team, led by Professors Jiang Hailong, Luo Yi, and Jiang Jun from the University of Science and Technology of China (USTC), has proposed an innovative approach.
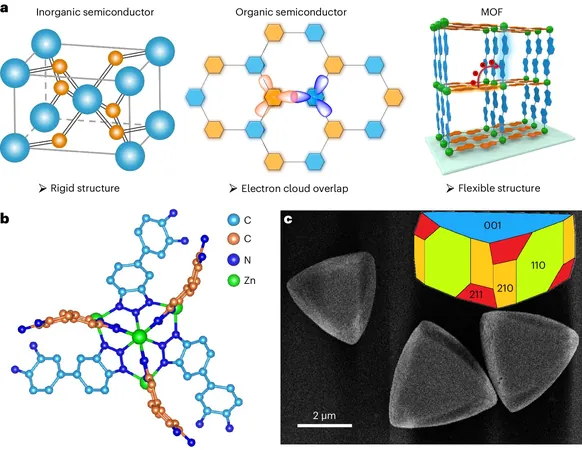
The Innovative MOF: CFA-Zn
The chosen MOF, designated CFA-Zn, incorporates closed-shell Zn2+ nodes linked by two flexible organic linkers that are chemically identical yet crystallographically independent. These linkers function as electron donor-acceptor pairs, while the stable Zn2+ structure maintains necessary chemical insulation between them. This sophisticated arrangement creates a flexible microenvironment mimicking that found in living plant cells.
Mechanism of Action
Upon exposure to light, CFA-Zn undergoes a structural alteration that stabilizes the excited-state electrons, significantly extending their lifespan and paving the way for enhanced overall water splitting efficiency. Prof. Jiang Hailong’s team has previously explored modifying the microenvironment surrounding catalytic centers, leading to adaptations in catalytic sites that achieved high selectivity for CO2 photoreduction to methane (CH4).









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)