A Breakthrough Discovery: New Organometallic Compound Defies 18-Electron Rule!
2025-07-15
Author: Benjamin
Revolutionary Findings in Organometallic Chemistry
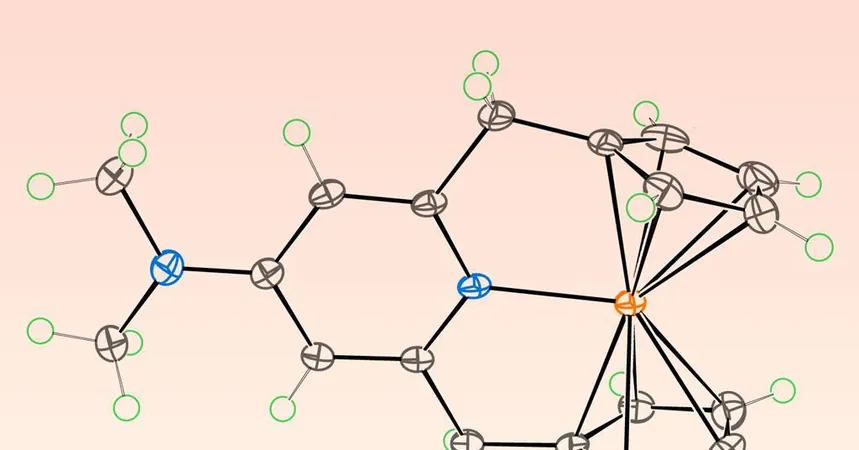
A groundbreaking new derivative of ferrocene has been discovered, boasting a staggering 20 valence electrons, and shattering the long-established 18-electron rule that governs organometallic compounds! This exciting revelation comes from a team of international researchers from Germany, Japan, and Russia, who are redefining the foundations of organometallic chemistry.
The 18-Electron Rule: A Brief Overview
Traditionally, organometallic compounds—which feature at least one metal-carbon bond—are believed to achieve maximum stability with 18 valence electrons. This principle has served as a cornerstone for chemists to assess compound stability and mechanistic pathways. Ferrocene, noted for its iron atom nestled between two organic rings, has long been a standard bearer for this rule.
Breaking Barriers: The Quest for 20 Electrons
The collaborative research effort questioned the rigidity of the 18-electron rule. Satoshi Takebayashi from the Okinawa Institute of Science and Technology shares, "We just asked if the rule could be breakable with this type of complex." With a novel ligand design, researchers successfully enhanced the electron count to an unprecedented 20 by enabling reversible coordination with an intramolecular pyridine.
Understanding the Science Behind the Breakthrough
The addition of electron-donating substituents, namely para-methoxy and para-amine groups, boosts the electron density on the pyridine nitrogen, facilitating the formation of a robust iron-nitrogen bond. Computational studies have shown that this unique configuration reduces the covalent interactions between the iron center and the cyclopentadienyl rings, thus accommodating the extra electrons.
New Chemistry, New Possibilities!
The 20-electron derivatives of ferrocene exhibit extraordinary redox properties, capable of reversible transitions among Fe(II), Fe(III), and Fe(IV) under mild conditions. The unexpected transition to Fe(IV) during oxidation was a thrilling surprise for Takebayashi, who highlighted that this was feasible due to the introduction of two additional electrons.
The researchers also created a dicationic species from ferrocene, a feat traditionally requiring harsh oxidizing conditions—now possible under significantly milder environments. David Mills, an inorganic chemist, remarked on the significance of this breakthrough, stating how challenging it typically is to form a ferrocene dication.
Looking Ahead: Expanding the Horizons of Chemistry
Future endeavors aim to investigate the catalytic properties of these innovative 20-electron ferrocene derivatives, with hopes that they could pave new paths in areas such as catalysts, pharmaceuticals, and advanced material science. The team is also eager to explore other unconventional compounds that push the boundaries of established chemical norms—highlighting that breaking the mold is essential for scientific progress!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)